Advocacy News

The ACR is your source for advocacy news impacting your practice, patients, and the rheumatology subspecialty. In addition to staying on top of ever-changing policies and political landscapes for our members, we are often invited to give statements, provide comments, or work with lawmakers to promote new policies.
For the most up to date news regarding ACR advocacy efforts, be sure to follow @ACRheumDC on X.
Advocacy Update from the ACR D.C. Office
View highlights from the ACR advocacy team’s efforts to respond to recent executive orders and government actions, equipping you with the information you need in the coming weeks and months.
October 23, 2025
This week, ACR Convergence provides the opportunity for an in-person DC update with live Q&A! In DC, Congress continues to grapple with the government shutdown, leaving physicians caught between serving Medicare patients or cancelling services they are unsure will be covered. Despite the shutdown, the administration is moving forward with the Inflation Reduction Act (IRA) provisions that aim to reduce the cost of treatments for Medicare patients.
Rheumatology on the Hill: The State of Play in Washington, DC
Tuesday, October 28 | 8:00–9:30 AM ET
McCormick Place Convention Center, Room W185A-D
Join the ACR advocacy team for the advocacy and policy update at ACR Convergence! This session will include:
- Current state of play in Washington, DC
- Policy issues impacting rheumatology
- ACR’s advocacy so far in 2025
- Advocacy opportunities to advance rheumatology policy
- 30 minutes of Q&A with ACR advocacy leaders and staff
Also, keep your eye out for advocacy activities and resources throughout McCormick Place illuminating the College’s work in specific policy areas, educating members on RheumPAC, encouraging applications to Advocates for Arthritis, and welcoming ACR and ARP member input and participating in advocacy.
The federal government shut down at the start of its new fiscal year (FY) on October 1 (FY 2026) because Congress has yet to enact a discretionary appropriations bill to fund the government. While the House passed a short-term continuing resolution (CR) on September 18 to continue the current levels of government funding through November 21, the Senate failed to pass this or an alternative stop-gap funding measure by the deadline. The resulting government shutdown has halted funding for all nonessential government programs and agencies until Congress acts.
Medicare Reimbursements
The Centers for Medicare and Medicaid Services (CMS) announced October 21 that it has instructed all Medicare Administrative Contractors (MACs) to lift a hold and begin processing claims dated October 1, 2025 and later for those paid under the Medicare Physician Fee Schedule. This marks a shift from earlier instructions on October 16 to all MACs to continue to temporarily hold claims with dates of service of October 1, 2025, which caused great concern among the medical community, then another instruction later that same day reversing the instruction and stating it would “continue to process and pay held claims in a timely manner.” To date, CMS says that no payments have been delayed as statute requires all claims to be held for a minimum of fourteen days, and this recent hold is consistent with that statutory requirement. Practices may continue to submit claims accordingly.
CMS also indicates that providers may opt to continue offering telehealth services that are no longer reimbursable under Medicare after their expiration on October 1. However, they are encouraged to issue an “Advance Beneficiary Notice of Noncoverage” to Medicare beneficiaries and consider holding claims pending potential Congressional action. It will require congressional action to retroactively cover telehealth services rendered on or after October 1, and there is no guarantee that Congress will take such action. CMS’ communications do not address this, nor provide assurance that providers will eventually be reimbursed for telehealth services under Medicare delivered during the shutdown.
In the past, after a government shutdown, Congress restored policies retroactively to the date of the shutdown and kept Medicare providers reimbursements whole for the services delivered during that period. However, the longer the shutdown, the harder it is to implement a retroactive policy. Unlike other programs that have previously lapsed, telehealth waivers have never expired before, creating a complete shutoff of telehealth coverage and reimbursement for Medicare fee-for-service beneficiaries. The ACR will continue to prioritize efforts to reinstate telehealth flexibilities, recognizing telehealth as a vital tool for rheumatologic care.
Tell Congress to bring back telehealth flexibilities through the ACR’s Legislative Action Center: Ensure Telemedicine Continues to Support Patient Care!
CMS released final guidance for the third round of IRA Medicare Drug Price Negotiation Program on September 30, 2025. The ACR supported the passage of this program, as it intends to lower costs for Medicare patients by allowing the government to negotiate prices for frequently used treatments. The final guidance for the third round applies to the Initial Price Applicability Year 2028 and includes new provisions for Medicare Part B drugs, which will be eligible for negotiation for the first time alongside Part D drugs.
The guidance revises how CMS will calculate total expenditures for Part B drugs, now incorporating data from Medicare Advantage plans. It also addresses how manufacturers will be supported in implementing the maximum fair price for drugs in 2026, 2027, and 2028. However, it only provides limited detail on the implementation, reserving that for future guidance.
CMS will select up to 15 additional drugs for negotiation (announcement of the candidate list by February 1, 2026) and begin negotiations in 2026. Negotiated prices from the third cycle will apply as of January 1, 2028. Prices for the second round of drugs will be released by the end of 2025. The ACR will continue to support the Medicare Drug Price Negotiation Program and keep membership updated on program changes and drug selection and pricing.
Contact your members of Congress today and urge them to support science-based vaccine recommendations and oppose efforts that threaten patient access to vaccines.
For More Details
Register for ACR advocacy office hours, where you have access to our congressional, regulatory, state, and private payer advocacy staff, so any question or concern you have will find the right ears.
Advocacy Update Archives
This week, Congress grapples over funding amidst the government shutdown. This has left Medicare physicians and patients unsure as to what services will be covered and researchers unable to submit grant applications to federal agencies as well as other areas of uncertainty. The administration announced additional fees for new H1-B visa applicants which would deter much needed growth in the medical workforce and a new prescription drug access portal was announced allowing manufactures to bypass tariffs.
Congressional Update: Shutdown
The federal government has shut down because a new fiscal year (FY) started on October 1 (FY 2026), and Congress has yet to enact a discretionary appropriations bill to fund the government. While the House passed a short-term continuing resolution (CR) September 18 to continue the current levels of government funding through November 21, the Senate failed to pass this or an alternative stop-gap funding measure by the deadline. The resulting government shutdown has halted funding for all nonessential government programs and agencies until Congress acts.
Certain essential government operations will continue during the shutdown including the disbursement of Social Security and Medicare benefits, as these are considered mandatory spending measures. The U.S Postal Service, which is entirely self-funded, will also continue mail processing and delivery. As of October 2, 32,460 federal employees at the Department of Health and Human Services have been placed on furlough, representing 41% of the agency's workforce. While the agency will continue essential operations, like administering Medicare benefits, and maintaining operations at the National Institutes of Health (NIH) biomedical research hospital, all administrative functions will cease at the NIH during the shutdown. The agency will not hold grant peer review meetings or advisory council meetings, will not issue new awards, nor host or attend scientific meetings for the duration of the shutdown.
The grants.gov website will remain operational albeit without technical support. The drawdown of funds should continue to be available, but technical problems and manual approval requirements could delay access. Reporting deadlines for grantees remain in place without extension. Additionally, submission deadlines for grant applications will not change, however NIH staff will not review applications or answer questions during the shutdown due to administrative leave.
Telehealth
Medicare telehealth flexibilities, which were implemented to expand access for patients during COVID-19, expired September 30. Affected telehealth provisions include equitable reimbursement for telehealth services, Medicare beneficiaries’ access to telehealth services without geographic or originating site restrictions, and reimbursement for audio-only visits. Patients and providers have gotten used to these flexibilities, and they have contributed to a substantial increase in telehealth utilization, but now Medicare telehealth has returned to being a rural-only benefit (as it is in statute). As such, Medicare patients cannot receive most telehealth services in urban areas. Medicare patients also need to come to an “originating” site (such as a hospital) to receive these services and can no longer receive most telehealth services from home.
Notably, Medicare Advantage plans (which cover more than half of the total Medicare population) can still employ flexible Medicare telehealth coverage policies. The restrictions only apply to traditional Medicare. Thus, coverage of telehealth services could differ for Medicare beneficiaries depending on what Medicare plan they are enrolled in.
Clinicians who chose to provide telehealth services to Medicare patients during the shutdown despite the expiration of these provisions do so at the risk of not receiving reimbursement. In the past, after a government shutdown, Congress restored policies retroactively to the date of the shutdown and kept Medicare providers reimbursements whole for the services provided. However, the longer the shutdown period, the harder it is to implement a retroactive policy. Unlike other programs that have previously lapsed, telehealth waivers have never expired before, creating a complete telehealth coverage and reimbursement shutoff for Medicare fee-for-service beneficiaries. The ACR will continue to prioritize efforts to reinstate telehealth flexibilities, recognizing telehealth as a vital tool for rheumatologic care.
Tell Congress to bring back telehealth flexibilities through the ACR’s Legislative Action Center: Ensure Telemedicine Continues to Support Patient Care!
Other Policies of note for rheumatology that also expired September 30
Conrad 30 waivers expired, which allowed J-1 foreign medical graduates to apply for a waiver of the two-year foreign residence requirement upon completion of the J-1 exchange program (visitor visa is for educational and cultural exchange programs designated by the Department of State). The ACR supports the maintenance and expansion of the Conrad 30 waiver program to allow more J-1 foreign medical graduates to apply for a waiver of the 2-year foreign residence requirement upon completion of the J-1 exchange visitor program.
Work geographic practice cost indices (GPCIs) also expired Medicare physician payments are adjusted geographically using GPCIs. Congress has repeatedly extended a 1.0 “floor” on the work component to protect rural providers from disproportionately lower reimbursement. Stakeholders have expressed concerns that without this minimum, physician services in rural areas would be disproportionately affected by lower Medicare payments. Medicare uses GPCIs to adjust physician payment rates under the Physician Fee Schedule for three factors: physician time/intensity, practice expense, and malpractice. Areas with higher costs (e.g. New York, San Francisco) get higher payments, and areas with lower costs (many rural or small-town regions) get lower payments, unless a “floor” is applied.
Since the early 2000s, Congress has repeatedly set a temporary 1.0 “floor” for physician time in rural/low-cost states (which must be reauthorized), so that doctors there aren’t paid less for the same service. The expiration of the “floor” means that rural rheumatologists will receive a drop in payments for treating Medicare patients, thus exacerbating the financial strain they are already experiencing and potentially creating access issues for rural patients.
From the Administration
New H-1B Visa Fees
Per the Proclamation entitled, “Restriction on Entry of Certain Nonimmigrant Workers” a new one-time payment of $100,000 must accompany an application for an H-1B visa beginning on September 21, 2025, paid by the prospective employer on behalf of foreign workers currently outside the U.S. The order directs USCIS and the Department of State (DOS) to deny approval of H-1B petitions or visas that aren’t accompanied by this payment. The order took effect on September 21, and is set to last for 12 months, unless extended or altered by future action.
It applies to foreign nationals outside the United States who would seek H-1B admission, unless the $100,000 fee is paid, and new H-1B visa petitions (for those not already in H-1B status) outside the U.S. The Department of Homeland Security (DHS) may waive the restriction for an individual, group, or industry if the hiring is deemed in the national interest and doesn’t pose a threat to U.S. welfare or security.
ACR is concerned that this fee will severely hamper the ability of institutions to recruit highly talented medical graduates from overseas. We are also concerned that it will exacerbate workforce shortages, particularly in underserved areas. As such, ACR recently joined the AMA in a letter that urged the Trump Administration to categorically consider H-1B physicians entry into the U.S. to be in the national interest of the country, and waive the new application fee, so that H-1B physicians can continue to be a pipeline that provides health care to U.S. patients.
TrumpRx: Direct-to-Consumer Prescription Platform
The Administration announced that Pfizer will offer Most Favored Nation (MFN) pricing for its drugs in the Medicaid program, i.e. aligning U.S. Medicaid prices with the lowest prices Pfizer offers in other developed countries. As part of the agreement, Pfizer also will participate in a new direct-to-consumer platform, TrumpRx.gov, where select drugs will be sold at steep discounts (relative to list prices) if patients pay cash. The discounts on that platform would not be uniform across all drugs. One example, tofacitinib (Xeljanz), is slated to be offered at a 40% discount through this direct channel. Only a limited set of Pfizer’s drugs are included in the initial TrumpRx launch list.
In return for participating in this platform, Pfizer is exempt for 3 years from the recently implemented tariffs on imported pharmaceuticals, contingent on their increasing domestic manufacturing and investment. The ACR is actively monitoring this development and its impact on the Medicaid program, access to treatments, and drug prices.
September–October Letters to Policy Makers
Physician Payment:
Medical Workforce:
- 9/9: Congress: The ACR endorses the bipartisan Resident Physician Shortage Reduction Act to expand Medicare-supported GME slots by 14,000 over 7-years
- 9/25: Department of Homeland Security, The ACR joins the AMA and other medical socities calling on the Secretary to exempt physicians from a recently announced $100,000 H-1B visa application fee.
- 9/29: The Administration - Department of Homeland Security: The ACR joins coalition calling for physician J, I & F visa holders to be exempt from proposed changes to duration of status rules.
- 10/2: Congress: The ACR endorses the Specialty Physicians Advancing Care in Rural Areas (SPARC) Act to establish a loan repayment program for specialty physicians practing in rural communities, and applauds Congressional champions upon reintroduction.
How Can You Take Action?
Contact your members of Congress today and urge them to support science-based vaccine recommendations and oppose efforts that threaten patient access to vaccines.
For More Details
Register for ACR advocacy office hours, where you have access to our congressional, regulatory, state, and private payer advocacy staff, so any question or concern you have will find the right ears.
This week, Congress is running up against the expiration of telehealth flexibilities on September 30. The administration welcomes input on the impact of noncompete agreements in medicine and the new CDC Advisory Committee on Immunization Practices (ACIP) meets for the first time.
Congressional Update
Telehealth Flexibilities to Expire September 30
Tell Congress to extend telehealth access today through the ACR’s Legislative Action Center: Ensure Telemedicine Continues to Support Patient Care!
Congress is facing a September 30 funding deadline to avert a government shutdown, but with the House in recess until October 1 it seems unlikely Congress will avoid funding disruption which means disruption to telehealth access. Medicare telehealth flexibilities, which were implemented to expand access for patients during the COVID-19 pandemic, will expire along with the funding. These telehealth provisions include equitable reimbursement and Medicare beneficiaries receiving telehealth services without geographic or originating site restrictions. Maintaining and extending these policies is a top priority for the ACR, as telehealth is an important tool for our members and their patients’ access to care.
While the ACR has advocated to extend telehealth flexibility long term, the House passed a short-term continuing resolution on September 18 to continue the current levels of government funding and extend telehealth flexibilities through November 21. The ACR urged the Senate to pass this stopgap measure to preserve telehealth access. Unfortunately, the Senate failed to pass that package as well as an alternative bill that proposed to keep the government open until Oct. 31. The ACR is urging the Senate to pass a stopgap measure to prevent disruption to telehealth access.
We hope you will join the ACR in urging Congress to take action to stop the uncertainty and secure permanent access to telehealth services, which will enable the full integration of this valuable resource into clinical rheumatologic care.
FTC Taking Comments on Noncompete Agreements
On September 4, the Federal Trade Commission (FTC) issued a Request for Information (RFI) asking for comments and data about employer non-compete agreements. The stated purpose is to better understand their potential harm through information on the “scope, prevalence, and effects” of these agreements. To inform possible future policy action, the FTC RFI is requesting further information from stakeholders that includes:
- Names of specific employers that impose non-compete agreements.
- Experiences of employees or former employees who are (or were) bound by non-competes: how it affected job changes, salary, career options, small business startups.
- Information on non-compete agreements in the healthcare sector (wages, access to services, quality, cost).
- Data on non-solicitation or non-recruitment clauses, and how these compare or interact with non-competes.
- How non-competes are used: Are they “all employees” or only higher paid/specialized roles, geographic scope, duration, and whether there are less restrictive alternatives.
ACR members are strongly encouraged to confidentially participate in this RFI, which could reduce the negative impact of noncompete agreements on healthcare professionals. Instructions for responding confidentially are included in the RFI. The deadline for responding is November 3.
Additionally, if you would like to work with the ACR advocacy team on an op-ed commentary detailing how a non-compete agreement has adversely impacted you, email the ACR’s advocacy team at advocacy@rheumatology.org.
New CDC Vaccine Advisory Committee Meets
The new members of the ACIP, the CDC’s vaccine advisory committee, met on September 18-19. The ACIP has been restructured under Health and Human Services Secretary Robert F. Kennedy Jr., including replacement of all of the members who served in previous years. This action has been covered in previous ACR D.C. updates.
During the first day of the two-day meeting, the committee voted not to recommend the combined MMRV vaccine for children under age 4. Instead, children under age 4 would be advised to get separate immunizations: one for MMR (measles, mumps, rubella) and one for chicken pox. Medical and public health experts have expressed concern about potential reductions in vaccine uptake if children must receive separate shots instead of a combined one. The committee initially voted to maintain coverage under the Vaccines for Children (VFC) program for both the combined MMRV and the separate MMR + chicken pox shots for children under 4.
On the second day of the meeting, the ACIP reversed the outcome of Thursday’s vote that would allow VFC coverage for the combined MMR and varicella vaccination, citing confusion among the committee about the wording of the recommendation. The committee also indefinitely tabled a recommendation for newborns to receive the hepatitis B vaccine.
The remainder of the meeting focused solely on the COVID-19 vaccine.
- Notably, the committee voted not to require state and local jurisdictions to require a prescription for patients to receive a COVID-19 vaccination. This decision came down to a 6-6 split, with the chair, Martin Kulldorff, casting the tiebreaking vote. Members of the committee engaged in a spirited debate on this recommendation, with those opposing the motion arguing that requiring a prescription would impose an undue barrier to access.
- The committee voted to recommend COVID-19 vaccinations to adults 65 and older, as well as to all individuals over the age of 6 months, following the process of “individual-based decision-making,” which calls for patients to speak with a clinician about “potential benefits and risks of vaccination,” while considering the patient’s risk factors for severe COVID infection.
- The ACIP recommended that the risks and benefits of the COVID-19 vaccine be provided, in writing, to patients and healthcare practitioners, including “at least six risks and uncertainties associated with the vaccine” as determined by ACIP.
While these recommendations do not immediately limit access to vaccines, stakeholders during public comment cautioned the ACIP against including risks known to be untrue or speculative. ACIP recommendations are non-binding and require approval by the Acting Director of the CDC.
How Can You Take Action?
Contact your members of Congress today and urge them to support science-based vaccine recommendations and oppose efforts that threaten patient access to vaccines.
For More Details
Register for ACR advocacy office hours, where you have access to our congressional, regulatory, state, and private payer advocacy staff, so any question or concern you have will find the right ears.
This week, Congress is back from August recess to face a funding deadline that includes the expiration of telehealth flexibilities on September 30. The administration’s tariffs that would have increased the cost of many biologics are struck down in court and the Center for Medicaid and Medicare Services (CMS) answers questions on the new Wasteful and Inappropriate Service Reduction (WISeR) prior authorization model.
Congressional Update
Telehealth Flexibilities Set to Expire September 30
Congress returned from August recess last week and now faces a September 30th funding deadline to avert a government shutdown. Notable for rheumatology, the Medicare telehealth flexibilities which expanded access for patients since their implementation during COVID-19 will expire unless Congress acts to extend them, as it did in its March funding legislation. These flexibilities include equitable reimbursement and the ability for Medicare beneficiaries to continue receiving telehealth services without geographic or originating site restrictions. It is a top policy priority for the ACR that telehealth remains an accessible tool for our members and their patients.
Extending telehealth long-term or for another short-term period will be a part of the broader appropriations process, which remains uncertain. Without a unifying policy goal or clear funding offsets, lawmakers in both parties are hesitant to pursue a comprehensive legislative effort. It is more likely that Congress will pass a short-term continuing resolution (CR) to extend the current levels of government funding and incorporate language that extends flexibilities for the use of telemedicine during the same timeline as they did in December 2024 and in March of this year.
While a short-term extension may maintain access to Medicare telehealth services, these temporary solutions fail to address the long-term sustainability and reliability of access and parity. The ACR supports making telehealth flexibilities and parity permanent which will enable the full integration of this valuable resource into clinical rheumatologic care. Tell your Congressperson to extend telehealth access through the Legislative Action Center today: Ensure Telemedicine Continues to Support Patient Care!
Updates on the Administration
U.S. Court Imposes Stay on Tariffs Impacting Pharmaceuticals
On August 29, 2025, the U.S. Court of Appeals for the Federal Circuit delivered a 7–4 decision upholding a decision by the U.S. Court of International Trade that most of President Trump’s sweeping tariffs that targeted imports (including pharmaceuticals) from Mexico, China, Canada, and the European Union—imposed under the International Emergency Economic Powers Act (IEEPA)—were not explicitly authorized by statute and therefore could not be imposed by law. Notably, the decisions allow the tariffs to stay in place until October 14 to give time for the administration to appeal to the Supreme Court, which the administration has signaled it will do.
For rheumatology, items like infusion chairs, sterile supplies, imaging equipment, diagnostic kits, ultrasound machines, and syringes used in rheumatologic care are or have components sourced from abroad for which tariffs raise acquisition costs for practices and hospitals. Additionally, a large share of biologic agents come from these countries as well.
As rheumatology professionals already operate under tight margins, the ACR opposes the threat of additional tariff-related costs which would make it harder to sustain infusion suites, purchase updated equipment, or maintain adequate staffing.
New FAQ Page Explaining CMS WISeR Model
As a follow-up to its recent announcement of the new WISeR Model, CMS has published answers to some of the most frequently asked questions concerning the WISeR Model on a new FAQ page. This six-year pilot program runs from 2026 to 2031 and targets Traditional Medicare with artificial intelligence-supported prior authorization to reduce spending on services deemed wasteful or of limited clinical value.
In the FAQs, CMS goes into greater detail on the planned implementation of the WISeR Model as well as how the model fits into CMS’s overarching goals concerning patient safety, data privacy, and ensuring Medicare savings. Notably, the FAQs discuss participant compensation and the denial and appeals process. It also discusses how the WISeR model specifically aligns with HHS Secretary Robert F. Kennedy Jr.’s and CMS Administrator Dr. Mehmet Oz’s pledge to fix the prior authorization system.
The ACR has sent a coalition letter to CMS asking to rescind the model on the grounds that it would actually increase the burden of prior authorization on providers.
August and September Letters
Barriers to Access:
8/4: ACR coalition calls for the Office of Inspector General (OIG) to further study the impact of step therapy on access to Part B treatments
8/12: ACR coalition calls for CMS to rescind the WISER model in Medicare fee-for-service
Physician Payment:
8/4:
ACR coalition calls for improved physician access to Medicare claims data
8/22: ACR coalition applauds introduction of the “Protecting Patient Access to Canter and Complex Therapies Act” to stabilize part B reimbursement
Access to Treatments:
8/29: ACR calls for the Senate Finance Committee to exercise oversight and address vaccine access issues in response to CDC staffing upheaval
Medical Workforce:
8/28: ACR coalition implores the Secretary of Education to maintain long-standing federal loan limit exceptions for borrowers pursuing a medical education.
Medical Research:
8/8: ACR and Arthritis Foundation request $11 Billion for CDC Arthritis Program in FY26 Appropriations
How Can You Take Action?
Contact your members of Congress today and urge them to support science-based vaccine recommendations and oppose efforts that threaten patient access to vaccines.
For More Details
Register for ACR advocacy office hours, where you have access to our congressional, regulatory, state, and private payer advocacy staff, so any question or concern you have will find the right ears.
This week, the Senate Appropriations Committee pushes back against cuts to research with a new funding bill, the ACR adds up canceled rheumatology grants, and members of Congress join the ACR in expressing concerns around policy to expand the use of prior authorization in Medicare.
Rheumatology Research Update
New Report Shows Idle Research Funds as Deadline Approaches
The Association of American Medical Colleges (AAMC) released a new report reflecting that while the National Institutes of Health (NIH) committed to almost $5 billion less to U.S. institutions in research grants this past year than the year before, the true shortfall in NIH funding far exceeds that gap due to the thousands of grants terminated mid-project, funds that have been frozen, and the inability for some institutions to draw down from these obligated funds. Over 90% of states experienced a reduction in overall NIH funding ranging from tens to hundreds of millions of dollars.
Congress’s fiscal year deadline is September 30. On its face, there could be a failure to spend the money allocated to the NIH by Congress, but as the ACR researchers know, every dollar terminated, not awarded in grant funding or made unavailable to institutions equates to fundamental research not conducted, next-generation investigators not trained, clinical trials not started, and the stalling of scientific progress. Urge Congress to protect NIH medical research funding. Sending a message through the Legislative Action Center takes just a moment. Act today!
ACR Assesses the Impact of Canceled Grants on Rheumatology Research
Ongoing disruptions in federal research funding at the NIH have had a significant impact on rheumatology research. The ACR has been monitoring the scope of disruptions and tracking their impact on rheumatology research awards as well as affected ACR/ARP members. Data pulled in mid-July identified 45 projects relating to rheumatic disease that have had their funding frozen or terminated, with only four identified for possible reinstatement. Over half of these affected grants were funded through the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). The total of all impacted rheumatology grants was $60 million, which includes $35 million of funding that remains undispersed to recipients. These disruptions in rheumatology research funding span 19 Congressional districts in 14 states.
Of the funded projects identified by the ACR as rheumatology topics, 40% of affected projects listed an ACR or ARP member as the Principal Investigator. The total value of the canceled studies among ACR/ARP members was $32 million, including $21 million of funding remaining undisbursed and spanning nine Congressional districts in eight states. As of mid-July, six grants led by ACR/ARP members had been terminated, the funding for nine grants is frozen, and three grants have been identified for potential reinstatement. However, the Congressional fiscal year and therefore this funding allotment technically expires September 30, leaving reinstatement in jeopardy.
The ACR will continue to monitor the federal research landscape and support the needs of the rheumatology research community. If you have been affected by a change, freezing, or cancellation in federal research funding, please contact advocacy@rheumatology.org to share your story.
Current Appropriations Bill Pushes Back on Cuts to Research
When Congress comes back in September, its first task will be passing appropriations bills, including funding for Health and Human Services, which oversees the NIH, CDC, and other health agencies and healthcare programs. The Senate has passed its health appropriation bill through committee, while the House has yet to act on this segment of appropriations (the House has passed their Defense appropriations bill).
The bill approved in late July by the Senate Committee on Appropriations includes $47.2 billion for the NIH’s base budget, which is $400 million or about 1% more than this year. That small bump is not something the ACR or biomedical research community would normally celebrate, but the president’s budget proposal would have cut the agency by $18 billion, or about 40%. The Senate draft bill also does not include the president’s budget proposal to reduce the CDC by over half.
Additionally, the ACR is pleased that the Senate bill also rejects a massive NIH reorganization proposed by the administration and the proposed cap on Facilities and Administrative (F&A) fees added to NIH grants, which would drastically lower overhead reimbursement that universities get for conducting research. These issues were brought to lawmakers by ACR volunteers during the Advocates for Arthritis Capitol Hill visits in May, who educated Congress on the devastating impact of cuts to research support. The Senate measure must still be approved by the full Senate and reconciled with a yet-to-be-written House appropriations bill. The ACR will advocate for passage of the bill.
Congressional Letter Shares ACR’s Concerns on Increasing Prior Authorization Use in Medicare
The Wasteful and Inappropriate Service Reduction (WISeR) Model will allow for expanded use of prior authorization in the traditional Medicare program. The model is set to begin on January 1, 2026, and run for six performance periods. It will apply to a list of outpatient procedures identified as vulnerable to fraud, waste and abuse, or inappropriate use for providers and patients in New Jersey, Ohio, Oklahoma, Texas, Arizona, and Washington. The ACR has long supported streamlining the use of prior authorization to reduce the burden on providers and decrease delays to care and opposes this model. Earlier this month, the ACR joined a Coalition Letter to rescind the WISeR model to share our specific concerns and recommendations with the Centers for Medicare & Medicaid Services (CMS).
In a separate letter from select members of Congress to CMS, a group of 17 Democrats requested more information about the WISeR Model. The letter, led by two RheumPAC-supported members, Reps. Suzan DelBene (D-Wash.) and Ami Bera, MD (D-Calif.), expresses concerns shared by the ACR that WISeR will “likely limit beneficiaries’ access to care, increase burden on our already overburdened health care workforce, and create perverse incentives to put profit over patients.” The letter also highlights how the administration has publicly recognized the problems with prior authorization and secured pledges from health insurers to curtail abuses of the practice. The lawmakers requested a response from CMS with details about the model’s scope, implementation, and beneficiary safeguards by September 1. The ACR hopes that CMS’s response to this request from Congress might shed light on the future of the model for our members and other stakeholders.
How Can You Take Action?
Contact your members of Congress today and urge them to support science-based vaccine recommendations and oppose efforts that threaten patient access to vaccines.
For More Details
Register for ACR advocacy office hours, where you have access to our congressional, regulatory, state, and private payer advocacy staff, so any question or concern you have will find the right ears.
This week, an important Arthritis Research program is on track to be reinstated at the Department of Defense, new tariffs could increase costs and decrease access to rheumatology treatments, and the administration increased the pressure on Diversity, Equity, and Inclusion (DEI) policies. We also provide a recent example of how RheumPAC contributes to ACR advocacy efforts, as well as what the Advocacy Fund is and how it allows corporate and organizational partners to support our work.
Department of Defense (DoD) Arthritis Research Program Reinstated in Appropriations Bill
When the House of Representatives passed the Department of Defense Appropriations Act on July 18, it included language reinstating $10 million for an Arthritis Research program within the DoD’s Congressionally Directed Medical Research Program (CDMRP). Within the DoD, the CDMRP is funded by Congress directly to address the unique needs of active service members.
Among Iraq and Afghanistan veterans, 95% of medical discharges are attributed to post-traumatic osteoarthritis. The CDMRP Arthritis Research Program aims to improve military readiness and retention by preventing arthritis and advancing treatments. This includes modifying training protocols and educating service members about ways to avoid high-impact injuries that could lead to post-traumatic osteoarthritis. In March, Congress passed a continuing resolution that defunded the CDMRP Arthritis Program. Following this decision, the ACR and Arthritis Foundation advocated zealously for the reallocation of this funding.
The ACR is encouraged to see members of the House Appropriations Committee reinstate this important program and acknowledge the impact of arthritis on service members. The ACR will continue to advocate for the passage of this funding with the Defense Appropriations Bill through the Senate, which is likely to vote in September.
How Tariffs Could Impact Rheumatology
On July 27, the European Union (EU) and the U.S. agreed to a trade deal designed to de-escalate a looming transatlantic trade war and establish a framework for more stable economic relations. A unified 15% tariff will now apply to most EU goods entering the U.S., including pharmaceuticals (except for certain generic drugs made in Europe). These tariffs could impact rheumatology by raising drug costs, disrupting global supply chains, impeding patient access to vital therapies, and decreasing funds for research and development of new therapies.
The extent of the tariffs’ impact is likely to vary depending on individual companies' manufacturing locations and mitigation strategies. The rapidly evolving nature of the administration’s trade policy also introduces further uncertainty. The ACR opposes any policy that negatively impacts drug prices, patient access to treatment, or the cost of providing high-quality care. As such, we are actively monitoring the implementation of these and any tariffs and assessing their impact on rheumatology.
Administration DEI Update
On July 30, Attorney General Pam Bondi issued a memo titled Guidance for Recipients of Federal Funding Regarding Unlawful Discrimination to all federal agencies. The memo applies to all federal funding recipients—including hospitals and clinics receiving Medicare, Medicaid, NIH grants, or fellowship funds—and puts DEI initiatives under civil rights enforcement scrutiny. The guidance emphasizes that any DEI initiative, regardless of intention, may now be found counter to Title VI, VII, or IX, and the Equal Protection Clause if it states explicit or proxy-based preference for protected groups.
The new guidance identifies several examples relevant to healthcare HR and programming it deems problematic:
- “Diverse slate” hiring policies (requiring protected group representation in candidate pools)
- Race- or sex-specific recruitment, scholarships, internships, or fellowships
- Programs based on “cultural competence,” “lived experience,” or geographic targeting serving as proxies for protected traits
- Affinity groups or trainings limited to certain protected groups, i.e., DEI workshops, are now flagged as potential violations
The memo includes non-binding recommendations to help institutions comply, such as incorporating explicit nondiscrimination clauses into grant agreements, monitoring third-party compliance, and terminating funding for perceived non-compliance.
Additionally, on August 7, President Trump signed an Executive Order titled Improving Oversight Over Federal Grantmaking, which directs the heads of Health and Human Services (HHS) and other executive agencies to designate a senior appointee to oversee grant-making processes and ensure that the projects selected to receive funds are in agreement with the administration’s priorities and that funds are not being used to promote racial discrimination, illegal immigration, or “sex binary denial”.
The ACR fervently believes in the value that diversity brings to the rheumatology workforce and to elevating the quality of patient care. The ACR will continue to monitor these developments and respond as new details emerge that allow us to better understand how implementation will impact rheumatologists, rheumatology care team members, and their patients.
The Impact of RheumPAC
RheumPAC, the ACR’s political action committee (PAC), is a critical educational tool, and it was recently put into action in a major way. When an initial version of the One Big Beautiful Bill Act (OBBBA) was released earlier this year, it included a harmful elimination of the Pass-Through Entity Tax (PTET) deduction, a portion of the tax code that allows private practice owners to deduct some state-level taxes on their federal returns.
Educating lawmakers about the impacts of removing the PTET deduction was crucial. Many legislators who ACR staff met with at RheumPAC events did not know that the provision had been included in the bill, or that the deduction benefited medical providers. The educational opportunities provided by RheumPAC-sponsored political events complemented the ACR’s formal lobbying efforts, like coalition letters to Congressional leadership.
See coalition letters to Congressional leadership >
The final version of the OBBBA preserved the full PTET deduction for private practice owners, meaning healthcare professionals were able to avoid a 1.5–5% tax hike that they might otherwise have incurred without the deduction. Without RheumPAC, the ACR would not have had a seat at the table to express concerns about this issue and advocate for the best interests of our members.
Learn more about RheumPAC and make your annual investment >
What Is the ACR's Advocacy Fund?
Legislators are just one part of the advocacy universe. While we leverage RheumPAC to educate lawmakers about our issues, it’s critical that we continue to expand our donor base and create pathways for more ACR/ARP members and patients to be involved with our advocacy efforts. That’s where the Advocacy Fund comes into play.
Launched in 2018, the Advocacy Fund is a powerful partnership between the ACR and rheumatology community stakeholders like private practices, state societies, and other provider organizations who want to support the ACR’s advocacy efforts but are not eligible to contribute to RheumPAC. Advocacy Fund dollars are more flexible and may be spent on a variety of advocacy-related projects as opposed to just supporting Congressional re-election campaigns.
An organization or corporation may want to support issues, a district or state important to the rheumatology community, but not align with supporting a specific candidate. The Advocacy Fund offers a powerful partnership between the ACR and corporate allies aimed at protecting, promoting, and strengthening the rheumatology community. For example, a portion of Advocacy Fund contributions go directly to supporting fellows in training and patient advocates attending the Advocates for Arthritis fly-in, a cost that RheumPAC dollars are federally prohibited from covering.
Learn more about the Advocacy Fund or contribute via a corporate or business credit card >
June and July Letters to Policymakers
Medical Workforce
- 6/4: Expressing concern with changes to student loan access in the One Big Beautiful Act
- 6/24: Expressing concern with limiting access to student loans and increasing barriers to pursuing medical education
Access to Treatment
- 6/13: Calls for Congress to address barriers to access to biosimilars and reimbursement (identical letter sent to the House)
- 6/18: Call to Reinstate Terminated CDC Vaccine Panel and Voicing Concern regarding the Impact of HHS' Actions on Vaccine Access
- 6/25: An open letter to the American public supporting vaccinations to protect against respiratory viral infections
- 7/31: ACR and state rheumatology society letter urging Secretary Kennedy to reinstate terminated members of ACIP and to reaffirm HHS's commitment to a science-driven process for guiding national immunization practices
Physician Reimbursement
- 6/23: Calls on Senate to establish a permanent inflationary update to the MFFS tied to the Medicare Economic Index
- 7/9: Calls for Congress to integrate registries in value-based models and improve access to claims data
How Can You Take Action?
Contact your members of Congress today and urge them to support science-based vaccine recommendations and oppose efforts that threaten patient access to vaccines.
For More Details
Register for ACR advocacy office hours, where you have access to our congressional, regulatory, state, and private payer advocacy staff, so any question or concern you have will find the right ears.
This week, the Centers for Medicare & Medicaid Services (CMS) released its Calendar Year (CY) 2026 Medicare Physician Fee Schedule (PFS) proposed rule, which includes recommendations related to Medicare physician payments and the Quality Payment Program (QPP). Also, Congress moves forward from the One Big Beautiful Bill Act and the ACR team pushes policy priorities for consideration for the September policy package.
2026 Medicare Physician Fee Schedule Proposed Rule Released
On July 14, 2025, CMS released its CY2026 Medicare PFS proposed rule, which includes proposals related to Medicare physician payment and the QPP. The suggested ule has a 60-day comment period. Final regulations will be issued on or around November 1 and unless otherwise noted, policies will be effective January 1, 2026. Among others, the ACR will provide comments on the following provisions.
2026 Medicare Physician Fee Schedule Proposed Rule Released
On July 14, 2025, CMS released its CY2026 Medicare PFS proposed rule, which includes proposals related to Medicare physician payment and the QPP. The suggested rule has a 60-day comment period. Final regulations will be issued on or around November 1 and unless otherwise noted, policies will be effective January 1, 2026. Among others, the ACR will provide comments on the following provisions.
Fee Schedule Provisions
Physician Reimbursement
- Separate updates for Qualifying Alternative Payment Model (APM) Participants (QP) and non-QP clinicians are proposed for 2026. This is the first year CMS will implement separate conversion factors based on QP status.
- The CY2026 qualifying APM conversion factor is projected to increase by $1.24 (3.83%) to $33.59, from the current $32.35. Similarly, the CY2026 nonqualifying APM conversion factor is projected to increase by $1.17 (3.62%) to $33.42, from $32.35.
- The change to the conversion factors reflects the temporary one-year increase of 2.5% included in the budget reconciliation bill signed into law on July 4 by President Trump.
- The overall reimbursement for rheumatological services is projected to increase by 4% for 2025.
Efficiency Adjustment
- CMS is proposing a -2.5% “efficiency adjustment,” which would apply to the work Relative Value Unit (RVU) and corresponding intraservice portion of physician time of non-time-based services.
- This would apply to all codes that are not based on time, such as evaluation and management (E/M) services, care management services, and services on the Medicare telehealth list.
- The efficiency adjustment would be every three years, with the next adjustment applied in CY2029, reflecting efficiency gains measured from 2027–2029.
Telehealth
- CMS is not proposing an extension to the waiver of geographic and “originating site” requirements for Medicare telehealth services that began during the COVID-19 pandemic and are in place through September 30, 2025, due to multiple extensions from Congress. Without additional congressional action, the waivers expire on October 1, 2025, and telehealth originating site requirements will go back to pre-pandemic limitations on patient location.
- In the 2024 PFS, CMS established a process that assigned HCPCS codes either a “provisional” or ongoing “permanent” status on the Medicare Telehealth Services List. CMS is proposing to simplify the review process for adding new telehealth services by removing the “provisional” and “permanent” categories and focusing on whether the service can be provided via telehealth. If finalized, all services added to the Medicare Telehealth Services List will be presumed to be permanent.
G2211
- CMS is proposing a minor adjustment to the G2211 complexity add-on code to allow it to extend its application to home or residence E/M visits, in addition to its current usage with office/outpatient E/M services.
Quality Payment Program
- CMS is proposing to maintain the performance threshold at 75 points for CY2026. They are proposing to maintain this threshold through the CY2028 performance period.
- CMS is not proposing to change the weights for the performance categories. The quality performance category will be weighted at 30% and the cost performance category will be weighted at 30%. The promoting interoperability and improvement activities performance categories will maintain their respective 25% and 15% weights.
- CMS is proposing to expand the portfolio of available MVPs and is revising the format of each MVP (including the ACR’s Advancing Rheumatology Care MVP) to categorize the quality measures by clinical conditions or episodes of care.
- CMS will be introducing a new format of the MVP tables in 2026 to stratify quality measures by clinical conditions and/or episodes of care for each MVP, identified as “Clinical Groupings.”
What Is Next for Congress?
In the formation of the One Big Beautiful Bill Act (OBBBA), Congress considered a number of the ACR’s policy priorities for inclusion in the legislation. While none of the ACR’s priorities passed with the final bill, another legislative package must pass before October 1 to fund the government and prevent a shutdown.
Stabilizing Physician Reimbursement
Language tying the conversion factor for the Medicare PFS to the Medicare Economic Index (MEI) to more appropriately adjust for inflation in 2026 and 2027 was included in the House version of the OBBBA. The ACR joined a medical specialties coalition letter to have this provision included in the final package. Ultimately, an adjustment for inflation failed to be included, but the bill added 2.5% to the Medicare physician pay for calendar year 2026. The ACR will advocate for long-term reforms to stabilize physician payments under Medicare to be included in the September legislative package, including automatic annual inflation adjustments. Ultimately, the balanced budget requirement needs to be repealed to allow physicians to be reimbursed at the actual cost of providing care to patients. The ACR will continue to advocate for that reform, but such a policy change has yet to be proposed by Congress and there is no legislative language to cite.
Reforming the Business Practices of Pharmacy Benefit Managers (PBMs)
Medicare reimbursement and PBM reforms have been a priority for the ACR and were discussed with lawmakers during the College’s Hill Day on May 6. Major reforms to PBM business practices included in the House OBBBA remained in the first Senate version of the bill. These policies promised to “delink” PBM income from the prices they negotiate for specific drugs and limit PBM income to “bona fide service fees.” Additional reforms increased transparency around drug formulary decisions that benefit any pharmacies affiliated with PBMs. Unfortunately, the provisions related to PBMs were removed from the version of the OBBBA that became law, but this recent language being considered by Congress is a great jumping off point. The ACR will continue to advocate for these important policy changes and urge congressional leaders to include such reforms in the September legislative package.
Telehealth Access
The issue of telehealth provisions has also been a recent legislative focus. In March 2025, Congress extended telehealth flexibilities once again through September 30, providing a temporary reprieve for millions of patients and clinicians, like rheumatologists, who have come to rely on virtual care. While this averted an access to care crisis, it failed to address the long-term uncertainty around access to telehealth, including whether payment parity for this form of care is here to stay. Temporary solutions undermine the full potential of telehealth, leaving providers in limbo and patients at risk of losing critical access to care. As the September package comes together and must either extend or eliminate broadened telehealth access, the ACR is advocating for permanent policy reform that sets reimbursement levels and parameters around use that allow providers to embrace telehealth’s full clinical potential.
How Can You Take Action?
Contact your members of Congress today and urge them to support science-based vaccine recommendations and oppose efforts that threaten patient access to vaccines.
For More Details
Register for ACR advocacy office hours, where you have access to our congressional, regulatory, state, and private payer advocacy staff, so any question or concern you have will find the right ears.
To kick off July, the healthcare community responds to the passage of the One Big Beautiful Bill Act, which included a historic reduction in federal funds for state Medicaid programs and a number of other provisions impacting rheumatology. Also, we follow up on the ACR’s advocacy around access to vaccines in the wake of the overhaul of the Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) by Health and Human Services (HHS) Secretary Robert F. Kennedy, Jr.
ACR Reacts to Passage of The One Big Beautiful Bill Act
Press Release: ACR Reacts to Passage of The One Big Beautiful Bill Act
In the May 20 and June 24 advocacy updates, the ACR examined the content of each chamber’s version of the One Big Beautiful Bill Act (OBBBA).
Language tying the conversion factor for the Medicare Physician Fee Schedule to the Medicare Economic Index (MEI) to more appropriately adjust for inflation in 2026 and 2027 was removed in the Senate version of the bill. The ACR joined a medical specialties coalition letter to have this provision included in the final package voted on by the Senate. While we were disappointed that an adjustment for inflation, or other extended reform, failed to be included in the final OBBBA, we are encouraged that the bill does includes a 2.5% increase to the Medicare Physician Fee Schedule for calendar year 2026.
One measure the ACR was concerned about would have eliminated the ability of certain pass-through entities, specifically those defined as specified service trades or businesses (SSTBs), to deduct state-level pass-through entity taxes (PTET) on their federal returns. Many dental and medical practices operate as pass-through entities, meaning their business income is taxed at the individual level. Pass-through entities provide a sustainable and competitive financial structure, especially for small rheumatology practices in rural or underserved areas. This measure was mitigated in the initial Senate version of the bill, but anything less than the current full deduction risked harm to our members. The ACR joined a coalition letter to voice concern regarding this tax provision to Congress and share how it would harm small businesses and jeopardize patient care across the country. The ACR is pleased that the provision eliminating or reducing the ability of practices to deduct PTET was not included in the final OBBBA.
Medicare reimbursement and pharmacy benefit manager (PBM) reforms have been a priority for the ACR and were discussed with lawmakers during our Hill Day on May 6. PBM reforms in Medicare Part D from the House bill remained in the initial Senate version. These policies promised to “delink” PBM income from the prices they negotiate for specific drugs and limit PBM income to “bona fide service fees.” There was also language increasing transparency around drug formulary decisions that benefit any pharmacies affiliated with PBMs. All of the provisions related to PBMs were dropped from the final version of the OBBBA. The ACR will continue to communicate our support for these important policy changes to congressional leaders.
Another policy area impacting rheumatology is student loan provisions that threaten to limit access to the healthcare workforce. The ACR joined a physician coalition letter explaining the threat to access to care a reduced cap on Grad PLUS loans poses. Eliminating the Graduate PLUS loan program or restricting aggregate lending will disadvantage the more than 40% of all medical students who use the programs, as they may no longer be able to afford medical school if required to borrow from the private loan market. We explained that physicians are very reliable borrowers who pay back their student loans, as evidenced by their extremely low default rates. In a follow-up letter, the ACR joined an expanded physician coalition on this topic and broader student loan repayment policies pertaining to the Public Service Loan Forgiveness (PSLF) Program, as updated language proposed excluding physicians and dentists from counting their residency and training years toward eligibility. The final OBBBA student loan changes include:
The Graduate PLUS program, which more than 40% of all medical students utilize, was eliminated in the OBBBA. For students who already borrowed a Grad PLUS Loan for June 30, 2026, and are still enrolled in their program of study, they will likely be grandfathered in to borrow for an additional three academic years, or until the completion of their program, whichever comes first. This places limits on Direct Graduate Loans, restricting graduate students to a lifetime total of $100,000 and professional students, like those pursuing medicine, to $200,000.
Parent PLUS Loans: Caps Parent PLUS loans at $65,000 per student.
Borrowers with outstanding federal student loans now have three years to transition from their current repayment plan to one of two New Repayment Plans:
- Standard Repayment Plan, which sets fixed payments for 10–25 years based on the borrowers' original balance, or
- Repayment Assistance Plan: Starting in July 2026, new borrowers can enroll in a new income-driven repayment plan. Under the plan, borrowers will pay back their loans over 30 years. The plan sets borrowers' payments at 1–10% of their income, depending on their income levels, with a minimum monthly payment of $10. Unpaid interest is waived under this plan, and any remaining balance will be forgiven after 30 years.
Finally, the OBBBA cuts federal matching requirements for state Medicaid programs. The threat to access to care posed by the reduction in federal funds for state Medicaid programs in the OBBBA is the basis for the ACR’s advocacy against this package. The ACR joined two physician coalition letters (see first letter, see second letter) opposing any cuts to Medicaid and any policies that limit access to Medicaid or treatment under the program for any of the 83 million Americans currently covered through the program. The OBBBA is estimated to reduce the amount of funding disbursed to state Medicaid programs over the next 10 years by $930 billion and risk 11.8 million patients’ access to the program. President Trump signed this bill into law on July 4.
ACR Advocacy on Vaccine Access
In June, HHS Secretary Robert F. Kennedy Jr. removed every member of the CDC Advisory Committee on Immunization Practices (ACIP) and replaced them with new members. Historically, nearly all of the panel’s recommendations have been accepted and used by insurance companies in deciding what vaccines to cover for patients.
If the ACIP's scientific credibility is undermined or its recommendations are rolled back, it could reduce access to vaccines for vulnerable populations, including immunocompromised rheumatology patients. Any disruption to ACIP processes could create uncertainty in these clinical decisions. Rheumatologists must stay updated on these evolving recommendations to ensure their patients receive the most appropriate protection, and the ACR will continue to advocate for rheumatologists and their patients.
In response to the ACIP changes, the ACR joined a medical specialty coalition letter to Secretary Kennedy, requesting that the original ACIP committee be reinstated: HHS: Vaccine Access – ACR Joins Medical Specialties Coalition Asking for Reinstatement of CDC ACIP Panel and Voicing Concerns Regarding the Impact of HHS Actions on Vaccine Access – 6/18/2025
Additionally, the ACR joined an open letter to the American public to promote public understanding and confidence in the use of evidence-based vaccines and immunizations: An Open Letter to the American Public: Barriers to Treatment – ACR joins AMA in reaffirming support for vaccination to protect against respiratory viral infections – 6/25/2025
This month, the ACR is broadening its advocacy by working with several state rheumatology societies to address vaccine access closer to home.
How Can You Take Action?
Contact your members of Congress today and urge them to support science-based vaccine recommendations and oppose efforts that threaten patient access to vaccines.
For More Details
Register for ACR advocacy office hours, where you have access to our congressional, regulatory, state, and private payer advocacy staff, so any question or concern you have will find the right ears.
This week, the Senate released its version of the One Big Beautiful Bill Act, which addresses the expiration of certain tax provisions from the 2017 Tax Cuts and Jobs Act, as well as other budgetary and spending changes. Earlier this month, Health and Human Services (HHS) Secretary Robert F. Kennedy Jr. removed and replaced every member of the Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP). Here we address the impact of these policies and actions on rheumatology and summarize the work of the ACR’s Washington, D.C., office on these issues.
Senate Reconciliation Update
In the May 20 advocacy update, ACR reviewed the content of the version of the One Big Beautiful Bill Act that passed the House of Representatives in May and was sent to the Senate.
The ACR is concerned about one measure in the House bill that would have eliminated the ability of certain pass-through entities, specifically those defined as specified service trades or businesses, to deduct state-level pass-through entity taxes (PTET) on their federal returns. Many dental and medical practices operate as pass-through entities, meaning their business income is taxed at the individual level. Pass-through entities provide a sustainable and competitive financial structure, especially for small rheumatology practices in rural or underserved areas. The ACR joined a coalition letter to voice concern regarding this tax provision to Congress and share how it would harm small businesses and jeopardize patient care across the country. This measure is mitigated in the Senate version of the bill, but is still concerning, as anything less than the current full deduction risks harm to ACR/ARP members.
In the Senate bill, PTET deductions would be limited to the greater of $40,000 or half of one’s PTET payments. Rheumatology practices rely on sound tax policy to remain financially viable and deliver high-quality care to the patients and communities they serve. The ACR is advocating that it be removed from the legislation before the Senate votes on the package.
The Senate bill increased cuts to Medicaid beyond the House version. As a reminder, the House Committee on Energy & Commerce committee draft cut $715 billion dollars from Medicaid by freezing state provider taxes, fees, and assessments that federal funds are required to match, and limiting State Directed Payments for services furnished after enactment from exceeding the Medicare payment rate. The Senate version reduces the 6 percent Medicaid provider tax in the House-passed reconciliation bill to 3.5 percent for states that have expanded the program, which further reduces the amount of state funds that federal funding would be required to match. The ACR opposes cuts to Medicaid and any policies that limit access to Medicaid or treatment under the program for any of the 83 million Americans currently covered through the program.
Language tying the conversion factor for the Medicare Physician Fee Schedule to the Medicare Economic Index to more appropriately adjust for inflation in 2026 and 2027 was removed in the Senate version of the bill. The ACR has joined a medical specialties coalition letter to have this provision included in the final package voted on by the Senate. This letter is being finalized and will be sent to the Senate this week.
Medicare reimbursement and pharmacy benefit manager (PBM) reforms have been a priority for the ACR and were advocacy issues discussed with lawmakers during our Hill Day on May 6. PBM reforms in Medicare Part D from the House bill remained in the Senate version. These policies will “de-link” PBM income from the prices they negotiate for specific drugs and limit PBM income to “bona fide service fees.” There is also language increasing transparency around drug formulary decisions that benefit any pharmacies affiliated with PBMs. The ACR will continue to communicate our support for these changes to congressional leaders.
If the Senate passes a new version, then that new bill goes back to the House, where the original package only passed by one vote in May. Some changes made by the Senate may upset the balance struck in the House and put the revised bill in question. The ACR will continue to stay engaged with Congress as they work on the package and will continue to voice our concerns over policies that threaten access to care, while supporting provisions that we have worked to see gain momentum to increase physician payment and reduce physician burden.
HHS Secretary Releases CDC Vaccine Panel
Earlier this month, HHS Secretary Robert F. Kennedy Jr. removed every member of the CDC Advisory Committee on Immunization Practices (ACIP) and replaced them with new members.
The ACR has joined a medical specialties coalition letter asking for reinstatement of the original ACIP committee and voicing concerns regarding the impact of the disruption of the committee’s work on vaccine access. This committee makes recommendations on how vaccines that have been approved by the Food and Drug Administration (FDA) should be used. Their recommendations traditionally go to the CDC director, but the CDC has no current director, and the committee’s recommendations have been going to Secretary Kennedy. Historically, nearly all the panel’s recommendations have been accepted and used by insurance companies in deciding what vaccines to cover for patients.
Secretary Kennedy said he removed the committee members because of conflicts of interest. The current policy requires that committee members declare any potential conflicts, as well as business interests, that arise during their tenure. They also must disclose any possible conflicts at the start of each public meeting.
On June 10, the American Medical Association (AMA) House of Delegates adopted an emergency resolution calling for reinstatement of all 17 ACIP members fired by Secretary Kennedy and a Senate Health, Education, Labor, and Pensions (HELP) committee investigation of the HHS secretary’s actions. Chairman of the Senate HELP committee, Bill Cassidy, MD (R), of Louisiana, also expressed concern about the future of the committee after the removal of the original members.
For More Details
Register for advocacy office hours, where you have access to our congressional, regulatory, state, and private payer advocacy staff, so any question or concern you have will find the right ears.
This week, the White House released an executive order on federal research and the Department of State paused new F and J Visa appointments. Also, the reconciliation package, officially titled the One Big Beautiful Bill Act, addressing the expiration of certain tax provisions from the 2017 Tax Cuts and Jobs Act and includes other tax and spending changes, faces an uncertain future as the Senate considers significant changes in response to conflicting priorities and public response to the House-passed package. Here we summarize the work of the ACR’s Washington, D.C., Office on these issues.
Freeze on F and J Visa Appointments
On May 27, U.S. embassies and consulates were ordered to pause scheduling new visa interviews for F and J visa applicants. The ACR is monitoring this temporary pause.
The Department of State has not officially communicated any changes yet. What we do know is that the suspension only applies to F and J visa applicants and does not affect applicants who have already scheduled visa interviews. This may affect recent medical school graduates who are foreign-born and educated outside of the U.S. who have not yet scheduled a visa appointment. Most medical residencies officially begin July 1, with orientations starting this month. International medical graduates (IMGs) without visas could miss their start date, putting their positions at risk and leaving institutions understaffed.
Even before this recent news, the Department of State mandated that all visa applicants disclose their social media accounts on the DS-160 forms and have been vetting social media accounts of visa applicants. Leadership from the Educational Commission for Foreign Medical Graduates (ECFMG), which serves foreign medical graduates entering the U.S., is attempting to gain an exception for physicians. ECFMG warned current residents who need to renew their visas not to travel outside the U.S. until the pause is ended.
New Executive Order on Federal Research
On May 23, President Trump signed an Executive Order calling for a restructuring of federal research policy to ensure what the administration refers to as “gold standard science.” The Order defines gold standard science as science conducted in a reproducible way; transparent; communicative of error and uncertainty; collaborative and interdisciplinary; skeptical of its findings and assumptions; structured for falsifiability of hypotheses; subject to unbiased peer review; accepting of negative results as positive outcomes; and without conflicts of interest. Further, the Order accuses the Biden administration of misusing scientific evidence in its crafting of public policies on climate change and public health guidance during the COVID-19 pandemic, among other policy initiatives.
An open letter in opposition to the Order, signed by more than 6,000 scientists, academics, physicians, researchers, and others, claims the order threatens scientific independence and infuses more political influence into the process. The letter asserts that the Order gives political appointees in charge of vetting scientific research the authority to “correct scientific information” and control the way it is communicated to the public, and the power to “discipline” anyone who violates the way the administration views science.
Reconciliation Update
The May 20 ACR advocacy update covered the content of the version of the One Big Beautiful Bill Act that passed the House of Representatives in May and was sent to the Senate. One measure that deserves further discussion is a provision that would eliminate the ability of certain pass-through entities, specifically those defined as specified service trades or businesses, to deduct state-level pass-through entity taxes on their federal returns. Many dental and medical practices operate as pass-through entities, meaning their business income is taxed at the individual level. Pass-through entities provide a sustainable and competitive financial structure, especially for small rheumatology practices in rural or underserved areas. The ACR joined a coalition letter to voice concern regarding this tax provision to Congress and share how it would harm small businesses and jeopardize patient care across the country.
Rheumatology practices rely on sound tax policy to remain financially viable and deliver high-quality care to the patients and communities they serve. Now that the bill has moved to the Senate, the ACR will advocate that it be removed from the next version of the legislation.
We expect to see a number of changes in the Senate version of the bill, but what these revisions will look like is difficult to predict. Conversations also continue between the Senate and the White House as the president is engaged in getting majority votes needed to pass the final package. Some majority legislators feel the bill needs to be moderated, while others feel the current bill doesn't go far enough and want to see bigger cuts to certain programs. The Senate majority leadership is facing an uphill climb to bring the legislation to a point of consensus.
If the Senate passes a new version, then that new bill goes back to the House, where the original package only passed by one vote. If anything changed by the Senate upsets the balance struck in the House in May, then passage of the revised bill in the House again could be in question. The ACR continues to stay engaged with the Senate to voice our concerns over policies that threaten access to care, while supporting provisions that we have worked to see gain momentum to increase physician payment and reduce physician burden.
May and June Letters to Policymakers
Barriers to Access
5/12:
Calls for removal of various burdensome Medicare regulations
Physician Payment
5/12:
Calls for removal of various burdensome MIPS regulations
Medical Workforce
5/13:
Addressing concern with proposals out of Education and Workforce Committee affecting medical student loans
5/20:
Expressing concern with proposed tax change that would negatively impact private practices
Research Funding
5/5:
Supporting the National Institute of Musculoskeletal and Skin Diseases (NIAMS) and express concern should it be consolidated into another division
5/8:
Requests $747 million funding increase for NIAMS in FY 26 Labor-HHS appropriations bill
5/19:
Calls for FY26 funding increase for the DoD Medical Research and restoration of FY25 program cuts
For More Details
Register for advocacy office hours, where you have access to our congressional, regulatory, state, and private payer advocacy staff, so any question or concern you have will find the right ears.
This week, the White House released an executive order on drug pricing, seemingly reviving the 2020 Most Favored Nation (MFN) proposal. Additionally, House committees release draft budgets with policy wins and big concerns for the rheumatology community. Finally, the Association of American Medical Colleges (AAMC) released a report on grants terminated by NIH. Here we summarize what the data from that report says and the work of ACR’s Washington, D.C. team on these policies.
New Executive Order on Prescription Drug Pricing
On May 12, the White House issued the "Delivering Most-Favored-Nation Prescription Drug Pricing to American Patients" Executive Order, which aims to lower prescription drug costs by implementing an MFN pricing model.
The order mandates a system where US prescription drug prices align with the lowest prices found in other developed nations. It also aims to allow manufacturers to sell directly to consumers at these lower prices. The order further directs the government to address foreign practices that may be inflating drug prices in the U.S. Should manufacturers not progress towards MFN pricing, the order proposes potential penalties, including drug importation and enforcement against anti-competitive behaviors. Ultimately, the goal is to reduce drug costs for American patients. However, specific policy details such as which drugs are included, and a timeline are not specified.
The first Trump Administration introduced a similar model in December 2020, focused on drugs covered by Medicare Part B. The ACR opposed this policy as it promised large price points and small margins for physicians, putting patients’ access to treatments at risk due to possibly severe financial ramifications, especially for small practices. The policy was rescinded before taking effect by the Biden Administration.
The ACR understands the implications this model may have on the rheumatology community and is closely monitoring any more details from the White House that may expand on how the policy will be implemented.
Details in the House Budget Impacting Rheumatology
For context, “reconciliation” is the legislative process that allows Congress to bypass the Senate filibuster and pass legislation related to federal spending with a simple majority vote in that chamber. Used by both parties at different times, this Congress is focused on a large reconciliation package to address taxes, border security, and other priorities of the administration. As we wrote about in March, both the House and Senate passed identical budget resolutions, which is the first step in the reconciliation process. But the proposed cuts outlined in those resolutions differed drastically between the chambers – a $1.5 trillion goal in the House versus $5 billion in the Senate. That gap creates tall hurdles between the chambers as lawmakers try to negotiate a unified package.
As such, the reconciliation process is still in the early stages. The House committees are releasing their budgets first and Medicaid/Medicare physician reimbursement, reforming the business practices of Pharmacy Benefit Managers (PBM), student loans, and other health policies are in play.
The House Committee on Energy & Commerce was tasked with finding $880 billion in savings over the next 10 years. The committee draft cuts $715 B from Medicaid by freezing state provider taxes, fees, and assessments that federal funds are required to match and limit State Directed Payments for services furnished after enactment from exceeding the Medicare payment rate. The committee's Medicaid policies also increase verification, renewal requirements, and eligibility redeterminations and increase allowable cost-sharing requirements. The ACR opposes policies that limit access to Medicaid or treatment under the program for any of the 83 million Americans currently covered through the program.
Other top-line rheumatology priorities from the Energy & Commerce draft include:
- Physician Reimbursement Reform: A new single conversion factor (CF) for Medicare Physician Fee Schedule services will be introduced in 2026, replacing the two separate CFs currently in use for physicians who are and are not employing Alternative Payment Models (APM). In 2026, the new singular CF would reflect 75% of the inflationary increase in the Medicare Economic Index (MEI), and in 2027, an additional 10% of that MEI increase.
- PBM reforms in Medicare Part D: “Delinking” PBM income from the prices they negotiate for specific drugs and limiting PBM income to “bona fide service fees.” Also, transparency for drug formulary decisions and coverage that benefits any pharmacies affiliated with PBMs.
Medicare reimbursement and PBM reforms have been a priority for the ACR and were advocacy issues discussed with lawmakers during our May 6 Hill Day. We will continue to monitor the progress of these policies and express support for these provisions making it into a final reconciled budget package.
In the Education & Workforce committee, one of the most consequential proposed changes is the restructuring of the federal student loan system in ways that risk making college and medical training less accessible. These policy changes include:
Eliminating subsidized Stafford loans, which currently allow low-income undergraduates to avoid accruing interest (and therefore further debt) while in school.
Imposing lifetime borrowing caps, including:
- $50,000 for undergraduates
- $100,000 for graduate students$150,000 for professional students
- $150,000 for professional students
- A combined $200,000 maximum for students and parents
The ACR fears that these limits will disproportionately harm potential medical students who rely on aid to fund their education. By restricting access to federal borrowing, it could be more difficult for people to return to education as many healthcare professionals do, a policy moving away from the needs of today’s potential healthcare labor market. As students seek to advance healthcare careers, continuous education, training, and access to affordable education opportunities are essential to success.
The language of the Resident Education Deferred Interest (REDI) Act (H.R.1202/ S.704) was also included in the Education & Workforce’s language. This policy allows physicians to defer student loan payments and the accrual of interest on their loans until they are finished with training, a change that recognizes that physicians do not enter the workforce upon graduation. The ACR continues to support this bill and the language becoming law.
AAMC Releases Report on Terminated Research Grants
On May 6, the Association of American Medical Colleges (AAMC) released a brief on the termination of NIH research grants since February this year showing that $1.9 billion in funding has been cancelled across hundreds of grants over the past few months. This reflects the experiences reported to us by ACR members who have had research grant funding terminated this year. AAMC drew from public data tracking grant cancellations at the NIH and the National Science Foundation since February.
The brief classifies the kinds of grants that were canceled and the amount of NIH funding lost in each state. AAMC tallied 777 grant terminations. Most of the terminated grants – roughly 90% – funded research and development, in addition to research training and career development. The AAMC brief found that the NIH’s Institutional Development Award program, which strengthens research efforts in states that have historically received less NIH funding, “lost a combined $75.4 million across 59 terminated grants.” Ninety-one of the canceled grants – amounting to $643 million in funding – supported 113 active clinical trials investigating topics such as HIV, lupus, mental health, and COVID-19.
Our team continues to advocate for adequate, reliable, and sustained research funding. If you have a personal experience to share or wish to advocate on this topic, please contact your members of Congress and let them know your story. Public research funding is budgeted by Congress to the NIH. As the only federal policymakers accountable directly to voters, they are in the best position to advocate a change of course to NIH officials and the administration.
Meetings and Events
Hill Meetings
- 114 ACR/ARP Hill Meetings May 6
- ACR Executive Committee meetings with:
RheumPAC Meetings/Events
- Dinner for Sen. Todd Young
- Breakfast for Rep. Debbie Dingell
- Dinner for Rep. Buddy Carter
All of the ACR letters sent to policymakers are available for review at any time.
For More Details
Register for advocacy office hours, where you have access to our congressional, regulatory, state, and private payer advocacy staff, so any question or concern you have will find the right ears.
This week, Congress has returned from recess and gone straight to work on a budget with long-term consequences for the rheumatology community. Additionally, the White House has released an executive order conditioning accreditation for academic institutions. Finally, the HHS reorganization continues and the ACR seeks answers from the Secretary on what the impact will be on the programs and projects that impact rheumatology. Here we summarize the work of the ACR’s Washington, D.C., office on these issues.
ACR Writes HHS Regarding Reorganization’s Potential Impact on NIAMS
On April 10, the Department of Health and Human Services (HHS) passback budget, which included potential changes to the National Institutes of Health (NIH), was leaked to the public. A passback budget is a preliminary proposal in which federal agencies and the White House Office of Management and Budget (OMB) negotiate discretionary funding and legislative priorities for inclusion in the Presidential Budget Request. It is not the final version of any budget or request. The College has reached out to the administration about the value of the work being done on rheumatic disease within the NIH and how to support it moving forward.
On May 5, leaders at the ACR and Rheumatology Research Foundation (RRF) co-authored a letter to HHS Secretary Kennedy to express support for high-value chronic disease research conducted at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and to proactively express concerns with the proposed reorganization of NIAMS should the proposal be implemented.
Highlighting the longstanding scientific partnership between the ACR and the NIH to advance rheumatic, musculoskeletal, and autoimmune disease research, the letter draws attention to the significant role of NIAMS in addressing chronic rheumatic and musculoskeletal diseases – namely arthritis – affecting millions of Americans. With the medical costs of rheumatic disease topping $140 billion per year, NIAMS research advances treatments to prevent and mitigate chronic disease progression, which in turn improves patient outcomes and prevents the need for more costly surgical interventions. The Make America Health Again (MAHA) commission has made its goal to understand and lessen the burden of chronic disease on the American people and the work of NIAMS pursues these goals each day.
The letter also alludes to a potential framework from the passback budget, which included consolidating NIAMS with two other NIH divisions: the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to form a new Institute of Body Systems Research. The ACR is concerned that this proposed NIAMS consolidation would crowd out available funding resources, thus posing the risk of significant reduction in rheumatic disease research necessary to improve the health of millions of Americans diagnosed with arthritis and rheumatic disease.
While the ACR recognizes the periodic need for institutions to review structure and strategy to remain successful, the letter implores Secretary Kennedy and HHS leadership to ensure that the essential elements of NIAMS are not lost in any large-scale institutional changes.
New Executive Order on Academic Accreditation
In an April 23 Executive Order (EO) titled Reforming Accreditation to Strengthen Higher Education, the White House directed the secretary of education to take various actions related to accreditors of academic institutions, including medical schools. Asserting that accreditors have “remained improperly focused on compelling adoption of discriminatory ideology, rather than on student outcomes,” the EO alleges that accreditors have been condoning discrimination in postsecondary programs. The EO also directs the attorney general and secretary of education to investigate and take action to cease unlawful discrimination that is “advanced” by DEI standards in medical schools and graduate medical education entities, among other institutions.
The directives in the EO will likely need to be implemented in some manner by the secretary of education before any effect can take place, likely through rulemaking. ACR takes seriously any threats to educational institutions and accrediting bodies and will monitor any proposed changes to accreditation that would impact members of the ACR and ARP. Institutions and accrediting agencies should also continue to closely monitor this matter for ongoing developments.
New Letters to Policymakers
4/7: Recommending $51 billion for the NIH in FY 2026 and reaffirming support for the agency
4/9: Congress called upon to address reimbursement barriers to biosimilar uptake
4/22: Encouraging the Department of Labor to close the Essential Health Benefits loophole
Review all of the ACR letters sent to policymakers.
For More Details
Register for advocacy office hours, where you have access to our congressional, regulatory, state, and private payer advocacy staff, so any question or concern you have will find the right ears.
This week, the House Ways and Means Health Subcommittee heard testimony from Colin Edgerton, MD, a member of the ACR Board of Directors, on biosimilars. Also, Congress is moving forward with the budget reconciliation process for fiscal year 2025 before recessing until April 28, including an $880 billion cut to Medicaid over the next 10 years.
Watch Testimony >
House Ways & Means Health Subcommittee Hears from ACR Leader
On April 8, the House Ways and Means Health Subcommittee held a hearing on lowering costs for patients by increasing access to biosimilar treatments. Biosimilars were introduced to the market as a lower-cost alternative to their original, reference biologics. However, insurers and pharmacy benefit managers (PBMs) have negotiated significant rebates from pharmaceutical companies in exchange for preferred placement of the treatment on formularies. These rebates are reflected in the manufacturers’ quarterly average sales price (ASP) reports to the CMS. Including the rebates in this calculus artificially lowers the ASP for the entire market. The result is that providers’ acquisition costs often substantially exceed Medicare and other private health plan payments.
During the hearing, Colin Edgerton, MD, RhMSUS, member of the ACR’s Board of Directors, testified about the need to modify the impact of PBM business practices on the pricing of biosimilars and adequately reimbursing for their infusion. Three other experts also testified and touched on the importance of small molecule research and development to produce new biosimilars, how far patient and provider knowledge of biosimilars has come, the value of competition and choice in the drug market, and the importance of adequate public research funding.
Specifically, Dr. Edgerton educated the committee on how the rebates negotiated between PBMs and manufacturers in exchange for biosimilars’ higher placement on formularies has led to providers being reimbursed less than the acquisition cost of biosimilars. Dr. Edgerton stated, “The ACR has been working to address a critical issue affecting rheumatologists, including in my own practice, which limits our patients’ access to biosimilars: the lack of adequate reimbursement for biosimilar treatments.” He further noted, “The gap between the cost of acquiring biosimilar treatments for administration to patients, and the amount we are reimbursed upon administering those treatments, is negatively impacting both physician practices and access to care for millions of patients suffering from chronic diseases.”
This is a policy priority for the ACR, as inadequate reimbursement limits access to care. The ACR and the Coalition of State Rheumatology Organizations (CSRO) co-lead the Underwater Biosimilars Coalition, whose primary objective is legislation that would modify the ASP calculation to mitigate or remove the impact of PBM rebates. Please share your thoughts with legislators about the impact of PBMs on the cost of treatments any time through the ACR legislative action center.
Fiscal Year 2025 Budget Moves Forward with Cuts to Medicaid
On April 2, the Senate majority released a budget resolution that lays the groundwork for significant program cuts, as it requires reducing the budget by at least $1.5 trillion. The House has voted to move forward on this version of the fiscal year 2025 budget resolution, and while both chambers will continue to negotiate the policy specifics, Medicaid, Medicare, the Affordable Care Act, and other valuable public health programs remain under threat. In previous budgeting cycles program cuts are listed as opposed to an amount, but the majority party has sidestepped aspects of the typical reconciliation process as they are under pressure from the White House to pass a budget bill by Memorial Day. The budget resolution would make permanent the 2017 tax cuts of the first Trump administration, using untested budget language —the “current policy baseline”—that would not require financial offsets.
This budget plan is an attempted compromise between the House and Senate. Both chambers previously advanced their own differing budget resolutions. but must pass identical versions to begin the reconciliation process. The original two bills from the chambers were far apart in both content and cost. Instead of resolving those differences in detail now, they have implemented this unorthodox strategy to give themselves more time. The resulting concurrent budget resolution reveals the House-Senate divide over the price tag, assigning different savings targets to each. The Senate committees are only tasked with finding $5 billion in savings, while House committees will take on their larger $1.5 trillion minimum goal. In most years, these numbers are aligned. In allowing the deviation, it appears that because reconciliation rules only apply in the Senate, only that chamber needs to meet its target.
Notably, these levels are floors, not ceilings, meaning lawmakers can cut more than the budget resolution requires, so we expect a reconciliation bill that is closer in size to the House’s vision of over $1 trillion in cuts. The House Committee on Energy & Commerce alone is tasked with finding $880 billion in savings over the next 10 years, cuts that are impossible to accomplish within the programs of that committee without cutting Medicaid.
Protecting access to healthcare is a policy priority for the ACR, as Medicaid provides access to care for 83 million vulnerable Americans. Support for Medicaid will be a topic for the Advocates for Arthritis Hill Day on May 6. Please tell legislators the importance of Medicaid to rheumatology patients today through the ACR legislative action center.
Meetings and Events
New Letters to Policymakers4/7: Recommending $51 billion for the NIH in FY 2026 and Reaffirming Support for the Agency
Review all of the ACR letters sent to policymakers at any time.
For More Details
Register for advocacy office hours, where you have access to our congressional, regulatory, state, and private payer advocacy staff, so any question or concern you have will find the right ears.
This week, the Department of Health and Human Services (HHS) dissolved several departments at the Centers for Disease Control and Prevention (CDC), including the two departments that oversee the lupus and arthritis programs. Congress confirmed Mehmet Oz, MD, to serve as secretary for the Centers of Medicare and Medicaid Services (CMS).
HHS Dissolves CDC Departments in Charge of Vital Arthritis and Lupus Programs
Last month, the administration announced a major restructuring of the HHS that will cut 20,000 full-time jobs and a reorganization of the department's 28 divisions within the CDC, CMS, the Food and Drug Administration (FDA), the National Institutes of Health (NIH), and other smaller organizations into just 15 divisions.
On April 1, the administration dismantled the Healthy Aging Branch of the CDC, which was responsible for arthritis-related research and programming. All employees of that branch were placed on administrative leave until June. The Epidemiology and Surveillance Branch at the CDC was also dismantled and the staff put on leave, including the lupus program staff that operated within that branch.
On April 3, the ACR and the Arthritis Foundation issued a joint press release reacting to these changes in the CDC and calling attention to the value of the programs within these divisions. This statement was just the first step in our advocacy efforts in reaction to this news, as the fate of the programs that were operated by the dismantled divisions remains unknown. We also plan to send letters to Secretary Kennedy at HHS and to Congress detailing the value of the work of these programs and the impact if they are reduced or eliminated. As our team learns more, these letters to policymakers in Congress and the decision makers at HHS will allow us to communicate in more detail the impact of these programs on our members and patients living with rheumatic disease.
Mehmet Oz Confirmed by the Senate
On April 3, the chamber voted 53 to 45 along party lines to confirm Mehmet Oz, MD, to lead CMS. Dr. Oz appeared before the Senate Health, Education, Labor & Pensions (HELP) Committee in March for his confirmation hearing. A former cardiothoracic surgeon, Dr. Oz will make and implement major decisions impacting the insurance coverage of more than 160 million people Americans enrolled in Medicare, Medicaid, or Affordable Care Act coverage.
As CMS administrator, Dr. Oz will wield significant power over most private health companies regarding decisions about who and what is covered by Medicare and Medicaid, including how physicians are paid through Medicare, which often sets a standard that commercial insurers follow. His role oversees more than $1.5 trillion in federal spending across Medicare, Medicaid, and the Affordable Care Act’s insurance exchanges.
Review the ACR letters sent to policymakers at any time.
Meetings and Events
Members of Congress: Hill Meetings
- Rep. Debbie Dingell (Arthritis Caucus Co-Chairwoman) re: the dismantling of CDC programs overseeing rheumatic disease programs
Partner Group Events
- Association of Women in Rheumatology (AWIR): Advocacy staff joined AWIR for their Hill Day Dinner
For More Details
Register for advocacy office hours, where you have access to our congressional, regulatory, state, and private payer advocacy staff, so any question or concern you have will find the right ears.
This week, the Department of Health and Human Services (HHS) announced a plan to cut an additional 10,000 full-time jobs and shut down several health agencies in a restructuring of the department. We also have our first fundraising report from RheumPAC. To close out the first quarter of 2025, below we recap all the legislation the ACR has endorsed this year, letters we have sent to policymakers, and meetings with legislators and stakeholders conducted by the ACR’s advocacy team so far in 2025.
Federal Update: HHS Announces Restructuring with 20,000 Job Cuts
On March 27, the administration announced a major restructuring of HHS that will cut 20,000 full-time jobs. The cuts include employees who have taken the Trump administration's previous offboarding and early retirement offers, plus an additional 10,000 jobs, to take the HHS workforce from 82,000 to 62,000. HHS is the umbrella agency that includes the Centers for Disease Control and Prevention (CDC), the Centers for Medicare & Medicaid Services (CMS), the Food and Drug Administration (FDA), the National Institutes of Health (NIH), and other smaller divisions.
The nearly 25% reduction in HHS staffing has had the most impact on the FDA, which has lost 3,500 positions, and the least impact on CMS, which has only lost 300. At the CDC, 2,400 employees will be let go as well as 1,200 at the NIH. Additionally, the proposed restructuring includes a reorganization of the department's 28 divisions into 15 by merging functions involving human resources, information technology, procurement, and policy; cutting the number of regional offices in half; and consolidating the department’s divisions. The restructuring will include the creation of a new Administration for a Healthy America (AHA).
These steep workforce reductions and drastic consolidations in functions at HHS are adding on to administrative efforts to cap indirect costs for NIH grants at 15%, remove Diversity, Equity, and Inclusion from HHS programs and resources, and bar public comments from certain HHS decisions. The ACR is deeply concerned by these endeavors and has spoken out against the cap on indirect research costs specifically. We are tracking how these consolidations and reductions in the HHS workforce might impact ACR and ARP members. Specifically, we will be monitoring any changes in Medicare and Medicaid coverage or reimbursement policies, as well as any changes to the FDA’s approval and regulation of prescription drugs through the Alliance for a Stronger FDA, of which ACR is a member.
RheumPAC First Quarter Fundraising Update
RheumPAC raised $25,269 from 74 ACR members in the first quarter of this year. This puts the only federal PAC representing rheumatologists in great shape to continue to support our congressional champions and build a Congress that supports the needs of the rheumatology community. That means 74 of your colleagues averaged contributions of $265 each. We want to see RheumPAC’s membership grow so more people are participating and we are relying on these generous donors less! Join your colleagues and make an investment today!
If you are new to advocacy or need a reminder, you can review all the FAQs. RheumPAC is nonpartisan and considers candidates based on their past support for ACR, their record on policies that directly impact rheumatology, or their position to further our goals in Congress. All funds disbursed are voted on and approved by the ACR’s volunteer-directed RheumPAC Committee.
First Quarter Advocacy Report (January 1–March 31)
ACR Endorsed Legislation
Dr. Lorna Breen Healthcare Provider Protection Reauthorization Act (H.R. 929 / S. 266)
- This first-of-its-kind law to support physician mental health and wellbeing was enacted in 2022 to improve the systems in which health workers are educated, trained, and practice. This bill would widen the program’s reach to more hospitals and health systems and prioritize funding to grantees that seek to reduce administrative burden, the primary driver of health workers’ burnout.
Medicare Patient Access and Practice Stabilization Act (H.R. 879)
- To zero out the 2.83% cut in Medicare physician payments implemented January 1, 2025, and provide a 2% inflation update, aiming to stabilize physician payments and protect patients’ access to care.
Reducing Medically Unnecessary Delays in Care Act (H.R. 2433)
- To require that all prior authorizations be made by a licensed physician who is board certified in the specialty relevant to the healthcare item or service requested.
Resident Education Deferred Interest (REDI) Act (H.R. 2028 / S. 942)
- To allow borrowers to defer their student loan payments interest-free while they are serving in a medical or dental internship or residency program.
Help Ensure Lower Patient (HELP) Copays Act (S. 864)
- To reduce barriers to treatment for patients by requiring health plans to count the value of copay assistance toward a patient plan’s cost-sharing requirements.
Conrad State 30 and Physician Access Reauthorization Act (H.R. 1585 / S. 709)
- To reauthorize and expand this program that allows foreign-born medical graduates to practice medicine in underserved areas. The bill extends the program for three years and increases current state allocations from 30 to 35 physicians per year. It also expands the number of waivers in states where demand exceeds that limit.
Letters to Policymakers by Policy Priority
Barriers to Access
3/7: Calls for Proposed HIPAA Security Rule updates to be retracted
1/27: Calls for streamlining prior authorization in Medicare Advantage
Physician Payment
3/10: Calling for the inclusion of a physician pay fix in the March government funding package
1/17: ACR comments on final 2025 Medicare Physician Fee Schedule
Access to Treatments
1/27: Calls for CMS to address inadequate reimbursement for biosimilars
Medical Workforce
Research Funding
3/5: Calling for the NIH to rescind 15% Cap to Facilities and Administrative Reimbursement
3/3: Reaffirms AHRQ support in the 119th Congress
1/31: Reaffirms NIH support in the 119th Congress
Meetings and Events
Members of Congress
Hill Meetings
- Doc Caucus (Republican Physicians of the House of Representatives)
RheumPAC Meetings/Events
- Breakfast for Rep. Mike Carey
- Lunch for Sen. Shelly Moore Capito
- Reception for Tuesday Group
- Meet and greet for Rep. Derek Tran
- Meet and greet for Rep. Mike Kennedy
- Lunch for Rep. Brian Fitzpatrick
- Lunch for Rep. Gus Bilirakis
- Meet and greet for Rep. April Delaney
- Meet and greet for freshmen GOP members hosted by Rep. John Joyce, MD
- Lunch for Rep. Neal Dunn
- Breakfast for Sen. Lisa Blunt Rochester
- Lunch for Rep. Mariannette Miller-Meeks
- Meet and greet with Rep. Rob Menendez
- Lunch for Rep. Kim Schrier, MD
- Reception for the Tuesday Group
- Dinner for Rep. Ami Bera, MD
- Reception for Sen. Lisa Blunt Rochester
Coalition Meetings
Underwater Biosimilars Coalition (January 6, February 10)
- To address insufficient reimbursements for biosimilars in the ASP calculation
Safe Step Act Coalition (January 8, February 12)
- To reintroduce legislation to address the impact of step therapy or “fail first” policies by payers on patients
All Copays Count Coalition (January 10, January 24, February 6, March 21)
- To support policies to count the value of copay assistance toward a patient plan’s cost-sharing requirements
Graduate Medical Education Coalition (January 22, February 25, March 25)
- To expand opportunities and positions for graduate medical education through funding for residency and training positions
Voice of Rheumatology (January 24, February 28, March 28)
- To gather rheumatology organizations and share information and strategies
Alliance for Transparent and Affordable Treatments (January 29, February 26)
- To support policies that reduce the negative impact of pharmacy benefit managers access to certain treatments and the cost of drugs to patients
Part B Access for Seniors and Physicians Coalition (February 4)
- Coalition to monitor and react to policies related to Medicare Part B, paying particular attention to factors impacting the calculation of the average sales price (ASP)
American Medical Association National Advocacy Conference (February 10-12)
- To understand the AMA’s policy goals and approaches for the year
Medical and Dental Specialties Coalition (March 20)
- Medical specialties meeting to find where policy priorities overlap and offer opportunities to work together
Regulatory Relief Coalition (March 12)
- To support legislation to reduce the negative impact of prior authorization requirements on physicians and patients
Partner Meetings
- Lupus Foundation of America (January 30)
- American Academy of Neurology (March 17, March 26)
- Arthritis Foundation (March 20, March 27)
- Society of Thoracic Surgeons (March 25)
- American College of Surgeons (March 26)
For More Details
Register for advocacy office hours, where you have access to our congressional, regulatory, state, and private payer advocacy staff, so any question or concern you have will find the right ears.
This week, multiple state legislatures are considering anti-vaccine policies, Congress is returning from a week of recess, and we outline the ACR’s work on health equity policy. Below, we summarize these happenings and the work of the ACR’s Washington, D.C. team.
State Update: Anti-Vaccine Legislation Emerging
Over the last few years, vaccines have been under increasing public scrutiny and have been the subject of intense and often clashing debate. As a result, we are increasingly seeing state legislation that could threaten patients' access to appropriate vaccines. In 2024, two states considered anti-vaccine legislation: Idaho and Montana. This year, that number is already 18 and likely to rise. Supporters of these bills are messaging them under the banner of “freedom of choice” and many of the policies have fines and jail time for non-compliance. Here is a breakdown of some of the legislation:
- Wyoming, Oregon, Oklahoma, South Carolina (Florida likely): Proposals to prohibit “hiring discrimination” against unvaccinated individuals.
- Iowa, Montana, and Idaho: Bills restricting or banning mRNA vaccines.
- Texas and Kentucky (Florida likely): Measures limiting mRNA vaccine use, with a specific focus on children.
- South Carolina: A proposal requiring providers to inform patients that the long-term safety of vaccines is "unknown."
- Texas: A bill requiring providers to give patients an information sheet created by an appointed committee on the potential short- and long-term side effects of mRNA vaccines.
- Arizona, Arkansas, Connecticut, Indiana, Minnesota, Mississippi, New Jersey, New York, Oklahoma, Oregon, Texas, and Virginia: Efforts to modify vaccine mandates and increase exemptions.
Finally, the Louisiana Department of Health recently banned state-sponsored vaccination events and ordered staff not to promote vaccination. For many uninsured or underinsured individuals, vaccination events are the only access they have to vaccination. These policies create access restrictions, which create a real danger to the chronically ill and those who care for them. The ACR will continue to voice appropriate concern where needed.
The ACR remains committed to ensuring that medical decisions remain between the patient and provider. We are working with our partners and the AMA to ensure that all patients living with chronic disease maintain access to FDA-approved vaccinations. Our team is also examining any legislation that would restrict an employer’s right to require vaccinations. If this policy gains traction, settings where employees would frequently be in contact with chronically ill and immunosuppressed patients may require an exemption to protect patients. Our members, hospitals, and physician offices need the freedom to choose to implement policies to protect themselves and the patient population they serve.
Health Equity Policy Update
The ACR recognizes that inequality and inequity are invisible undercurrents impacting the lives of many of our members and patients. The ACR condemns all acts that cause marginalization, discrimination, or harm to any person based on race, ethnicity, age, gender identity and expression, socioeconomic status, sexual orientation, religion, or disability. The ACR pledges to be a leader for inclusion and change for our members, trainees, staff, and patients.
Understanding the root causes and impacts these inequities have on patients and their health and finding effective solutions to lessen inequities is needed to improve access to care. From lupus morbidity and mortality to arthritis disability, and most recently to COVID-19 burden, severity, and deaths, people of color have suffered disproportionately. Physicians and healthcare professionals are bound to protect the health of all of humanity.
ACR supports:
- Initiatives that diminish racial and ethnic disparities for patients with rheumatic diseases, including care delivery and clinical trials.
- Research and government funding to identify, recognize, and reduce racial, ethnic, geographic, and socioeconomic disparities in rheumatic disease diagnosis, care delivery, and outcomes.
- Targeting funding for research and the evaluation of providers’ implicit bias and developing strategies and policies to address this.
- Recognizing the central role of social determinants on health disparities and health outcomes and the mobilization of resources and research funding to better understand and address these disparities.
- Increasing funding for rheumatology research workforce diversity.
- Reallocating unused visas for International Medical Graduates (IMGs) to ensure durable immigration status for those medical professionals.
- Additional programs that would expand access to durable visas for qualified healthcare professionals.
2025 ACR Policy Work in Health Equity
The Pediatric Subspecialty Loan Repayment Program (PSLRP) specifically addresses the shortage of pediatric specialists in underserved communities by encouraging pediatricians to pursue additional subspecialty training, despite the lower compensation compared to general pediatric practice, through loan forgiveness opportunities in exchange for service in these geographical areas. By providing loan forgiveness, this helps offset the decreased salary and helps incentivize the additional years of training and subsequent delay in earnings. After the advocacy of ACR and other medical specialties, this program was finally funded in FY2022 by the 117th Congress for the first time since its authorization by Congress in 2010. With the approved $15 million, it is estimated that approximately 100 two-year awards will be funded this year.
While this is an important first step, the ACR supports funding this program at $30 million per year. Additionally, the ACR continues to advocate for refining this program to better meet its original intent after HRSA has improperly weighed practice settings and other factors that favor non-rheumatology specialists.
The Conrad 30 program retains IMGs who complete their residency and fellowship training programs in the U.S. on a J-1 visa, but wish to stay and practice here. Without a Conrad 30 or similar waiver, these visas require IMGs to return to their country of origin for at least two years before applying for another visa or green card. The Conrad 30 program provides an exemption to these visa holders and allows U.S.-educated and -trained physicians with a J-1 visa to enter the American medical workforce directly upon the completion of their residency if the IMG practices in a medically underserved community. This helps reduce the current physician shortage in under-resourced areas.
Unfortunately, the number of waivers has been limited despite the increasing healthcare workforce shortage. This is why ACR has endorsed and sent letters in support of the Conrad State 30 and Physician Access Reauthorization Act (S. 709/H.R. 1585) to both the House and Senate. This legislation extends the Conrad 30 program for three years and increases each state’s allocations from 30 to 35 physicians per year.
On the state front, the ACR continues to support legislation that would expand access to fellowship opportunities and to service based loan forgiveness programs. Service-based loan forgiveness programs are designed to increase access to care for vulnerable populations. The ACR is supporting S.B. 130 in Georgia. It would expand access to service-based loan forgiveness programs to fellows. It would also make each fellowship slot eligible for $10,000 per year in state funding, payable to the designated institution. This is the most serious effort by a state in recent memory to address workforce shortages and increase patient access. We are hopeful that other states will adopt this approach.
What Has the ACR Advocacy Team Done So Far in 2025?
March Letters to Policymakers
Congress
- 3/13: Supporting the reintroduction of the Help Copays Act to prohibit the use of accumulator policies by health plans
- 3/10: Call to include fix for 2025 Medicare physician pay cuts in the appropriations package
- 3/3: Call for the Agency for Healthcare Research and Quality to be fully funded
Letters to National Institutes of Health (NIH)
Letters to the Department of Health and Human Services (HHS)
You can contact your Members of Congress directly through the ACR’s Legislative Action Center.
Meetings and Events, March 13–25
Congressional Doc Caucus (Republican Physicians in the House of Representatives)
Coalition and Partner Meetings
- Part B Access for Seniors and Physicians Coalition
- Medical and Dental Specialties Coalition
One-on-one meetings with:
American Academy of Neurology
Society of Thoracic Surgeons
- Arthritis Foundation
- All Copays Count Coalition
- Graduate Medical Education Coalition
For More Details
Register for advocacy office hours, where you have access to our congressional, regulatory, state, and private payer advocacy staff, so any question or concern you have will find the right ears.
This week in Washington, D.C., the Senate passed an appropriations package to avert a government shutdown. This package extended telehealth flexibilities through September 30, 2025, but it also cut 57% from one of the two federal medical research programs with dedicated arthritis funding. Also, Dr. Mehmet Oz, the potential new head of CMS, appeared before Congress. Here, we summarize these happenings and the work of the ACR’s Washington, D.C., team.
Congressional Update: The Appropriations Package’s Impact on Rheumatology
Telehealth Extended through September
Congress extended Medicare’s telehealth flexibilities through March 2025 during its 2024 lame-duck session, providing a temporary reprieve for millions of patients and clinicians, like rheumatologists, who have come to rely on virtual care. Once again, Congress has extended telehealth flexibilities temporarily, through September 30. While this averts a crisis, it fails to address the long-term uncertainty around access to telehealth, including whether parity is here to stay. Temporary solutions undermine the full potential of telehealth, leaving providers in limbo and patients at risk of losing critical access to care. The ACR supports permanent policy reform that sets reimbursement levels and parameters around use that allow providers to embrace telehealth’s full clinical potential.
Congressionally Directed Medical Research Program Cuts
When the Senate passed the appropriations bill Friday night, they passed a reduction to the Department of Defense’s (DoD) Congressionally Directed Medical Research Program (CDMRP) from $1.5 billion to $650 million. Created and sustained by Congress, the CDMRP competitively funds hundreds of projects each year at both DoD labs and non-DoD research institutions, including academic institutions, to study everything from cancer to battlefield wounds to suicide prevention and, since fiscal year 2024, arthritis.
As a part of the Defense Health Research Consortium, a group comprised of more than 60 research organizations, the ACR called on the Senate to undo the $859 million cut and preserve this crucial medical research funding prior to voting on a continuing resolution (CR). Arthritis is the second leading cause of medical discharge for the U.S. Army behind battlefield wounds. This research aims to prevent premature retirement due to preventable injury to retain talent and increase readiness. The goal of the funds is also to assess the root of the increased prevalence of rheumatoid arthritis in the armed services population versus the general population. Now that the cuts have passed, it is unclear how that will impact specific programs within the CDMRP.
What Is Next for Medicare Reimbursement?
In December 2024, as yet another cut to Medicare reimbursements approached, the Republican leadership promised the physician community that they would address the cuts in the next appropriations bill in March 2025, with an additional bump to make up for the months that the cuts were in place. Then, in February, the Republican Doctors’ Caucus reassured the physician community that H.R. 879, The Medicare Patient Access and Practice Stabilization Act, which would increase the conversion factor of the Medicare Physician Fee Schedule by 4.83%, would be included in the appropriations package being put forward by the Republican leadership in March.
As we saw, the appropriations bill was introduced and passed without this language. The ACR has scheduled a meeting with the House Doc Caucus on March 19 to share our frustrations, understand the avenues forward, and discuss the next steps. In the meantime, please contact your lawmakers about the impact of these cuts to physician services.
Administration Updates
Dr. Oz Appears Before the Senate
Dr. Mehmet Oz appeared before the Senate Health, Education, Labor & Pensions (HELP) Committee on Friday for his confirmation hearing for administrator of CMS. A former heart surgeon, he became best known as a TV personality after “The Dr. Oz Show” ran for 13 seasons. At CMS, Dr. Oz would oversee health insurance for around 150 million Americans enrolled in Medicare, Medicaid, or Affordable Care Act coverage. As CMS administrator, he would also wield significant power over most health companies through decisions about who and what is covered by Medicare and Medicaid. During the hearing, Dr. Oz did not address whether he would oppose cuts to Medicaid. He specifically cited Medicare Advantage (MA) plans, which have been a concern for ACR/ARP members whose patients’ access to treatments have been restricted after signing on to MA plans. Dr. Oz did add that he would like to see restrictions around the use of prior authorization in MA plans, a policy change the ACR supports and is actively advocating for. Dr. Oz is expected to win confirmation.
Other Healthcare Nominations
On Thursday, Senate committees voted to advance the nominations of Marty Makary, MD, MPH, poised to lead the Food and Drug Administration, and Jay Bhattacharya, MD, PhD, set to helm the National Institutes for Health, for a full Senate vote. The nomination of Dave Weldon, MD, to the Centers for Disease Control and Prevention was withdrawn Thursday amid understanding there was not enough support from the Senate for him to be confirmed.
What Has the ACR Advocacy Team Done So Far in 2025?
Endorsed Legislation Introduced
The ACR signed on to endorse the reintroduction of the Resident Education Deferred Interest Act (REDI Act), as noted in the press releases from the original bipartisan co-sponsors Brian Babin (R-TX-36) and Chrissy Houlahan (D-PA-6). This legislation makes medical education more affordable by deferring the accrual of interest on loans while physicians and dentists are still in residency and training.
You can contact your Members of Congress directly through the ACR’s Legislative Action Center.
For More Details
Register for advocacy office hours, where you have access to our congressional, regulatory, state, and private payer advocacy staff, so any question or concern you have will find the right ears.
This week in Washington, D.C., Congress is counting down to a March 15 appropriations deadline with dire consequences for the rheumatology community. Without legislative action, not only will the government shut down, but the current Medicare telehealth flexibilities will expire. Also, a potential new head of the NIH appeared before Congress, and the public has slightly less say in policy changes made by HHS after the Richardson Waiver is rescinded. Here, we summarize these events and the work coming out of the ACR’s Washington, D.C. office, including the meetings and activities of our advocacy team and RheumPAC.
Congressional Update
Will Telehealth Make It to April?
In the final legislative push of 2024, Congress extended Medicare’s telehealth flexibilities during the lame-duck session until March 31, providing a temporary reprieve for millions of patients and clinicians like rheumatologists who have come to rely on virtual care. This averted an immediate crisis but failed to address the long-term uncertainty around access to telehealth, including whether parity is here to stay. Temporary solutions undermine the full potential of telehealth, leaving providers in limbo and patients at risk of losing critical access to care. The ACR supports permanent policy reform that sets reimbursement levels and parameters around use that allow providers to embrace telehealth’s full clinical potential.
Currently, uncertainty is gripping the system. If you see a patient today and want to see them back in a month, will telehealth be an option? We do not know if Medicare will reimburse for a telehealth visit in April. While Congress seemingly agrees on maintaining access to telehealth, they are less united on whether those visits should share full parity with in-office visits. Crucially for the broader appropriations legislation in which the telehealth extension would be included, lawmakers are not coming together around the other major factors of the appropriations package that must be agreed on by midnight on March 14. These items include the length of time the funding package should cover, the total amount of funding for military and non-defense programs, and other policy specifics. If Congress fails to make a deal before this deadline there is a two-week buffer, since the current telehealth flexibilities are in place through March 31. There would be two weeks between a shutdown and a change in access to telehealth visits.
Additionally, in February, the Republican Doctors’ Caucus assured the physician community that their members would not vote for an appropriations package that does not also include a mitigation to the 2025 cuts to the conversion factor by including the language of H.R. 879, The Medicare Patient Access and Practice Stabilization Act. This legislation would fully offset the 2.8% cut in the Medicare Physician Fee Schedule (MPFS) implemented on January 1, 2025, and add a 2% payment update to physician services provided after April 1.
Contact your lawmakers today about addressing these cuts to physician services in this week’s appropriations package >
The last two funding packages have seen the Republican majority fail to gather enough votes to pass a package without Democrat support, and today we saw a continuing resolution pass the House with only one democratic vote. That legislation extends telehealth through September but supports none of the ACR’s other priorities. The current legislation does not contain any policy addressing the cuts to Medicare reimbursements. Now, the Senate will consider this legislation. Senate Republicans will need to convince at least 8 Democrats to vote with them to pass this package to avert a government shutdown. The ACR is still advocating for a funding package that includes H.R. 879 and an extension of the current telehealth flexibilities. We are also preparing materials and information for our members to prepare for the impact if the bill and telehealth extension do not pass.
Contact your lawmakers today about preserving access to telehealth >
Administration Updates
New NIH Leader Appears Before the Senate
This week, Dr. Jay Bhattacharya appeared before the Senate Health, Education, Labor & Pensions (HELP) Committee for his confirmation hearing to lead the NIH. The Stanford health economist got attention during the COVID-19 pandemic when as a principal behind one of the first public declarations questioning the lockdown policies being instituted worldwide. “The Great Barrington Declaration” argued against lockdown measures in favor of letting low-risk individuals build up herd immunity while older, sick people self-isolated. Federal scientists and many others criticized the document. Dr. Bhattacharya’s hearing comes as the agency is reacting to layoffs, resignations, restrictions on grants, and plans to restrict funding, which have concerned many ACR/ARP members. Dr. Bhattacharya did not contradict the administration’s recent proposal to cap indirect costs for NIH grants at 15% and is expected to win confirmation.
HHS Rescinds the Richardson Waiver
The Department of Health and Human Services announced that it is rescinding its policy inviting public input on rules and regulations related to public property, loans, grants, benefits, or contracts, citing costs and inefficiencies that “impede the Department’s flexibility to adapt quickly to legal and policy mandates,” according to the notice. Known as the “Richardson Waiver,” this policy was implemented by Elliot Richardson, the Secretary of HHS in 1971, to follow notice-and-comment procedures for certain actions exempt under the Administrative Procedure Act (APA). Moving forward, HHS will follow a notice-and-comment process only as required by the APA.
This means that now health agencies no longer need to go through the notice and comment process for many policy changes around grants and benefits. This includes new rules that have been suggested by the administration, such as potentially adding in work requirements to Medicaid or redrawing how the NIH funds research. Such changes can be implemented faster without the Richardson Waiver in place, so the ACR will be monitoring HHS actions closely on behalf of our members.
What Has the ACR Advocacy Team Done So Far in 2025?
Letters to Congress
- 2/3: Reaffirm support for the NIH to the 119th Congress
- 2/10: Endorsing legislation to reverse the cuts to Medicare reimbursement and add additional increase starting in April 2025
- 2/20: Ask for a $1.77B funding increase for the NIH, prohibiting changes to the NIH Facilities and administrative cost reimbursements (Sec. 224), and any other sweeping changes by the administration
- 2/28: Call to include HR 879, the Medicare Patient Access and Practice Stabilization Act, in forthcoming government funding package to offset current MPFS cuts and provide an inflationary update to payment
- 3/3: Reaffirm support for the AHRQ to the 119th Congress
Note: You can also contact your representative and senators directly through the ACR’s Legislative Action Center.
Letters to National Institutes of Health (NIH)
Letters to the Centers for Medicare and Medicaid Services (CMS)
- 1/17: On final 2025 Medicare Physician Fee Schedule
- 1/27: To address inadequate reimbursement for biosimilars
- 1/27: To streamline prior authorization in Medicare Advantage
- 1/27: To ask to preserve certain Inflation Reduction Act policies and reform prior authorization in Medicare Advantage
Meetings and Events this week (March 6–12)
Coalition and Partner Meetings
- Part B Access for Seniors and Physicians Coalition
- Medical and Dental Specialities Coalition
- Arthritis Foundation
- Step Therapy Reform Coalition
- Regulatory Relief Coalition
RheumPAC Events
- Lunch for Rep. Kim Schrier, MD
- Reception for the Tuesday Group
- Dinner for Rep. Ami Bera, MD
For More Details
Want to talk about a policy or share an issue that is not on our radar? Register for advocacy office hours, where you have access to our congressional, regulatory, state, and private payer advocacy staff so any question or concern you have will find the right ears.
This week, we saw a new budget coming out of Congress and the administration put out orders of impact to the rheumatology community. Read below for additional updates and information regarding actions by the new administration, including the meetings and activities of our team and RheumPAC.
Administration Updates
On February 26, the administration released the Making America Healthy Again by Empowering Patients with Clear, Accurate, and Actionable Healthcare Pricing Information executive order. This measure requires transparency around healthcare prices, particularly of insurers and hospitals, including prescription drug prices, with the aim of reducing healthcare costs overall by increasing competition through the ability for patients to price shop. This directive requires the departments to update their enforcement policies to ensure compliance and will mean that healthcare entities must disclose actual prices, not just price estimates.
The administrative actions pertaining to research and funding did not see movement this week. Below is the status chart. For more details, see last week’s update below (dated February 25).
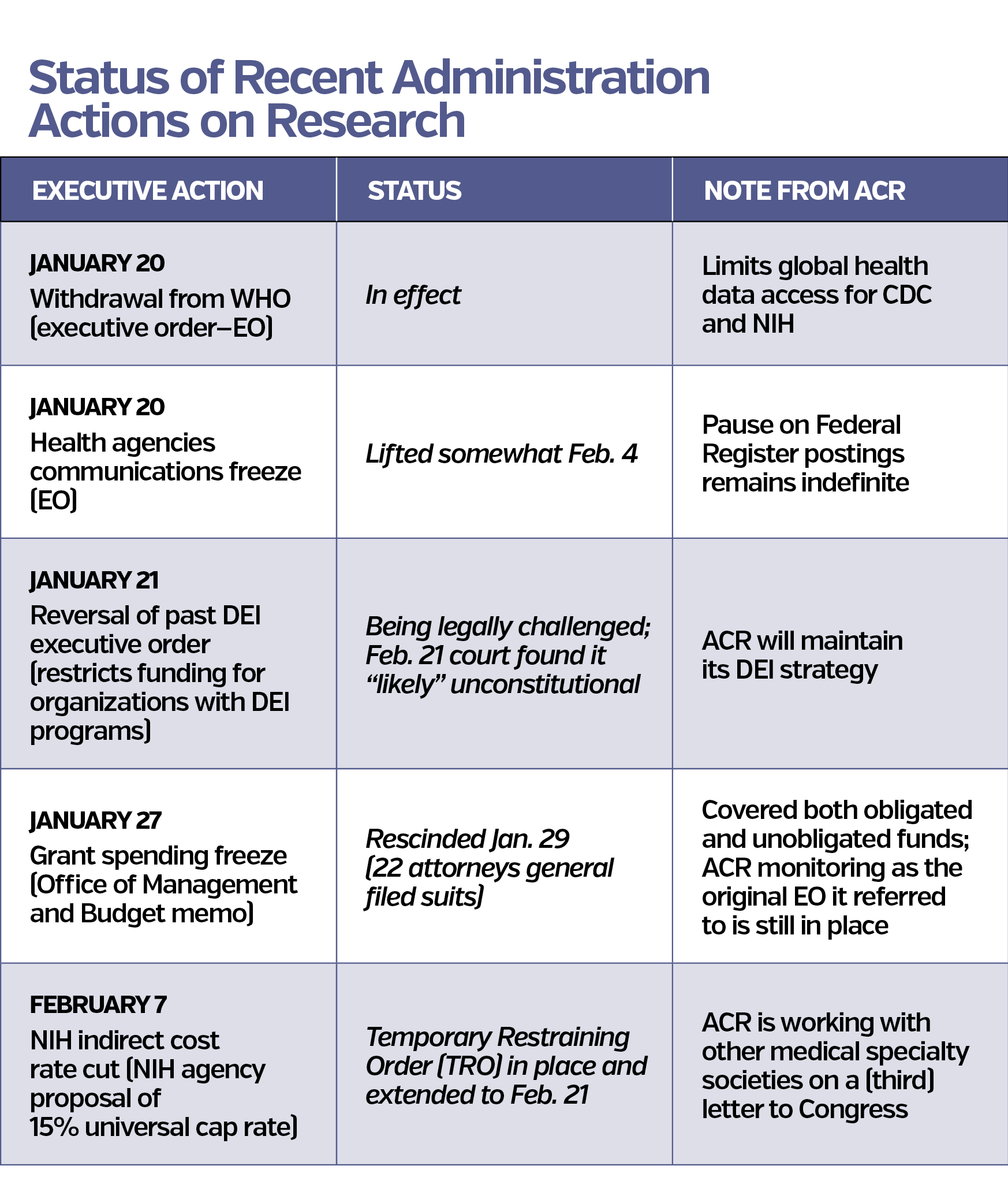
Congressional Update
Congress adopted its budget legislative proposal on February 25, weeks prior to the shutdown deadline on March 15. The package calls for $2 trillion in spending cuts, some of which could potentially impact Medicaid and other healthcare programs. The Senate passed its own resolution last week that would authorize around $340 billion in spending fully offset by corresponding spending cuts. The two chambers must pass a common budget resolution to move forward with the reconciliation process.
Currently, some key ACR policy priorities are missing from this package. Earlier this month, the Republican Doctors Caucus assured the AMA that members would not vote for a budget package that doesn’t include H.R. 879, The Medicare Patient Access and Practice Stabilization Act. This legislation would fully offset the 2.8% cut in the Medicare Physician Fee Schedule (MPFS) implemented on January 1, 2025, and add a 2% payment update to physician services provided after April 1. Currently, telehealth flexibilities under Medicare will expire at the end of March if not extended by Congress again as they were in the last budget cycle. If these measures are not included in the upcoming budget bill, the ACR will advocate for a standalone healthcare bill to address the necessary policies that must be addressed before these March deadlines.
What Has the ACR Advocacy Team Done?
Meetings and Events this week (February 26–March 4)
On February 26, the Energy and Commerce Health Subcommittee hosted a hearing titled An Examination of How Reining in PBMs Will Drive Competition and Lower Costs for Patients to examine the impact of the business practices of Pharmacy Benefit Managers on healthcare costs.
Watch Hearing
Coalition and Partner Meetings
- Voice of Rheumatology Alliance
- P B Access for Seniors and Physicians Coalition
- Medical and Dental Specialties Coalition
- Arthritis Foundation
RheumPAC Events
- Breakfast for Sen. Lisa Blunt Rochester
- Lunch for Rep. Mariannette Miller-Meeks
- Meet and Greet with Rep. Rob Menendez
- Lunch for Rep. Kim Schrier, MD
Want More Details?
The ACR advocacy team is keeping track of these actions, legal challenges, and the interplay between the executive actions and possible legislative intervention. We would be happy to talk to you more in-depth about a policy or hear about an issue that is not on our radar during our advocacy office hours.
Register for Office Hours >
During advocacy office hours you have access to our congressional, regulatory, state, and private payer advocacy staff, so any question or concern you have will find the right ears. There is no pressure to come with any previous policy knowledge or experience. This benefit of ACR/ARP membership simply grants more access to the advocacy staff. We want to hear from you!
New Administration, New Approach
Since January 20, we have seen a new approach to federal policymaking that necessitates modifying our approach to advocacy and communication with ACR/ARP members. It starts with increasing communication about what is happening here in Washington, D.C., to keep you abreast of new policies coming out of Congress and the administration and their impact on the rheumatology community. We will also summarize the work coming out of the ACR’s Washington, D.C., office, including the activities of our advocacy team and RheumPAC on behalf of the College.
Our approach to advocacy regarding executive actions is still evolving. While there may be an urge to loudly declare our position, to meaningfully impact the implementation of policies or work toward their reversal requires targeted strategies and open lines of communication with the administration. Please understand that the ACR will stand up for our members and their patients through the policy process. To this end, we expanded our 2025 Health Policy Statements to reflect the College’s support for:
- Applying science and data to healthcare policymaking
- Immunizations and vaccinations
- Transparency around Medicare Advantage plans
- Expanded access to visas for physicians to address workforce shortages
- Guardrails around private equity's investments in healthcare to ensure quality and affordability
Read below for additional updates and information regarding actions by the new administration.
Administration Updates and the Impact on Rheumatology
Executive Orders of the Second Trump Administration
With every new presidential administration, there are changes, which are consistent with a “normal” transition period.
Every president since George Washington has initiated a series of executive actions within hours of assuming office. These come in the form of “executive orders,” and they serve a few purposes. First, they are a vehicle to outline the overall stance of the incoming administration. Second, and in line with that positioning, such orders sometimes reverse executive orders made by previous presidents. Third, they can serve as broad rulemaking and guidance for the federal agencies under the authority of the executive branch. Executive orders may be used by all presidents, but these actions are subject to legal interpretation. In some cases, they have been deemed to exceed presidential authority and overturned. In other cases, they move forward.
Specific actions by the new administration have caused confusion and concern for some ACR/ARP members. These include a freeze on publishing pending regulations in the Federal Register, a temporary halt to external agency communications, and a federal hiring freeze, which could impact healthcare workers. These steps allow an incoming president to gain control of core functions of government, including political appointments and staffing. With the change of administration generally comes the departure of about one thousand personnel; those positions take time to fill.
Other executive actions stray from precedent but are repeats from the first Trump administration, like the NIH proposal to cut indirect costs by capping the rate at 15% which came out on February 7. In 2017, the first Trump administration proposed a 10% cap on indirect cost rates, which was blocked by the House and Senate Appropriations Committees in that Congress. This reflects the historical bipartisan support the NIH has had, and the ACR will continue to lobby Congress for support for the NIH. The ACR wrote to Congress on February 20 asking to preserve Sec. 224, which protects reimbursements for administrative costs.

Make America Healthy Again
The ACR advocates for specific policies to improve patient outcomes. Through this lens, our team will engage Robert F. Kennedy Jr. in his new capacity as HHS Secretary and whoever takes the helm of CMS to elevate the critical role rheumatologists and care team members play in the lives of patients and in the communities they serve.
On February 13, the administration expanded on its healthcare plans by releasing the Make America Healthy Again (MAHA) Executive Order. This measure establishes a MAHA Commission to address chronic disease, particularly in children, and promote healthy lifestyles and better nutrition. The ACR is hopeful this will present an opportunity to engage with policymakers in areas where we can collaborate to address juvenile rheumatic disease.
MAHA also emphasizes preventive care, vaccine transparency, environmental health, and integrative medicine to address chronic diseases and healthcare costs. The ACR has supported vaccines and immunization schedules as laid out by the CDC, and we are in touch with our colleagues at pediatric specialty societies about areas where MAHA vaccine transparency policies might impact vaccine access. The ACR will also continue to monitor access to treatments of comorbid conditions in rheumatic disease, such as obesity and depression, and MAHA policies impacting medications for those conditions.
In alignment with ACR policy priorities, MAHA supports rural healthcare through student loan forgiveness and telehealth investments aiming to increase provider numbers in underserved areas. The ACR hopes to work with the administration to hopefully make the current telehealth flexibilities permanent.
Congressional Update
The 119th Congress has convened and been largely eclipsed by the work of the White House. In the House, the Republicans hold an even slimmer majority this Congress than the last and continue to differ within the party on certain approaches. Members of the House are already thinking about their 2026 elections. In the Senate, there is a new majority party and a new Republican Senate Majority Leader, John Thune. The Republican majority is 53 seats to the Democrats’ 45, with two independents serving this Congress. The filibuster rule remains in place, so 60 votes are required to pass any legislation that is not “germane to tax, spending, and the debt limit.” Those financial policies only require 51 votes to pass the Senate.
The first big bill for rheumatology in this Congress is The Medicare Patient Access and Practice Stabilization Act (H.R. 879) led by Representatives Greg Murphy, MD (R-NC), and Jimmy Panetta (D-CA). This legislation would fully offset the 2.8% cut in the Medicare Physician Fee Schedule implemented on January 1, and add a 2% payment update to physician services provided after April 1. The ACR is advocating to have this measure included in the upcoming budget bill, which must come together before the current extension expires on March 15.
Congress has begun work on a March legislative package to keep the government funded past the March 14 deadline. The House of Representatives is considered “the purse,” so the budget bill must start there. The Republican Doctors’ Caucus has assured the AMA it will not vote for a package that doesn’t include H.R. 879, the House Democrats have said they will not step in to give Speaker Mike Johnson the votes he could not find from his majority party in the last two budget packages, and there are multiple other interests and factors. In addition to H.R. 879, the ACR would like to see reforms to pharmacy benefit manager business practices included in the March package that were stripped from the December budget package as well as reforms to prior authorization and our other 2025 ACR Policy Priorities.
What Has the ACR Done So Far in 2025?
Letters to Congress
- 1/31: Reaffirm support for the NIH to the new 119th Congress
- 2/10: Endorsing Legislation to reverse the cuts to Medicare Reimbursement and add additional increase starting in April 2025
- 2/20: Ask for a $1.77B funding increase for the NIH, prohibiting changes to the NIH Facilities and administrative cost reimbursements (Sec. 224), and any other sweeping changes by the administration
You can also contact your representative and senators directly through the ACR’s Legislative Action Center.
Letters to the Centers for Medicare and Medicaid Services (CMS)
- 1/17: On final 2025 Medicare Physician Fee Schedule
- 1/27: To address inadequate reimbursement for biosimilars
- 1/27: To streamline prior authorization in Medicare Advantage
- 1/27: To ask to preserve certain Inflation Reduction Act policies and reform prior authorization in Medicare Advantage
2025 Meetings and Event Activities (through February 27)
- American Medical Association National Advocacy Conference
- Coalition and Partner Meetings
Arthritis Foundation
Lupus Research Alliance
Safe Step Coalition (re: step therapy reform
Regulatory Relief Coalition (re: prior authorization reform)
All Copays Count Coalition (re: copay accumulator reform)
Voices of Rheumatology Alliance
Part B Access for Seniors and Physicians Coalition
Medical and Dental Specialties Coalition
- RheumPAC Events
Breakfast for Rep. Mike Carey
Lunch for Sen. Shelly Moore Capito
Reception for Tuesday Group
Meet and greet for Rep. Derek Tran
Meet and greet for Rep. Mike Kennedy
Lunch for Rep. Brian Fitzpatrick
Lunch for Rep. Gus Bilirakis
Meet and greet for Rep. April Delaney
Meet and greet for freshmen GOP members hosted by Rep. John Joyce, MD
Lunch for Rep. Neal Dunn
Want More Details?
The ACR advocacy team is keeping track of these actions, legal challenges, and the interplay between the executive actions and possible legislative intervention. We would be happy to talk to you more in-depth about a policy or hear about an issue that is not on our radar during our advocacy office hours.
Register for Office Hours >
During advocacy office hours you have access to our congressional, regulatory, state, and private payer advocacy staff, so any question or concern you have will find the right ears. There is no pressure to come with any previous policy knowledge or experience. This benefit of ACR/ARP membership simply grants more access to the advocacy staff. We want to hear from you!
As ACR President, Carol A. Langford, MD, MHS, mentioned in her message to members earlier this month, the ACR is endeavoring to maintain ongoing communication with members in these rapidly changing times to ensure that you can continue to provide excellent care to your patients.
To that end, we want to highlight ways that the ACR’s advocacy team is working to respond to recent executive orders and government actions and equip you with the information you need in the coming weeks and months.
- The ACR’s advocacy team is actively monitoring unilateral executive actions that are shifting the health policy landscape daily. The ACR’s team in Washington, D.C. will be providing regular updates summarizing the happenings in D.C. and what they mean for you and your patients.
- ACR/ARP members are invited to join the ACR’s advocacy team on February 20 and February 27 at 11:00 AM ET via Zoom for office hours to learn more about our team’s efforts on behalf of you and your patients, ask questions about ACR advocacy, and find out how you can get involved from home. You will receive a confirmation email with video call information once your membership status has been verified by the ACR team.
Register for Office Hours > - Additionally, you may continue to contact your lawmakers through the ACR's Legislative Action Center. The ACR maintains campaigns that emphasize the importance of data and evidence-based policy making as well as broad access to public health information and guidance to address the health needs of the U.S. population.
As we continue to monitor these issues, please do not hesitate to reach out to advocacy@rheumatology.org with any questions or concerns.
Advocacy Priorities
See an overview of the ACR’s Policy Priorities for the U.S. Congress, federal agencies, and state governments.
Press & Public Statements
View the latest rheumatology news, including press releases and public statements about current advocacy issues affecting ACR and ARP membership in the Press Room.
Social Media
Be sure to follow the ACR on Facebook, X, Instagram, and LinkedIn. For advocacy specific updates, follow @ACRheumDC on X. ACR often interacts with lawmakers, posts pictures, circulates articles, and more. Get engaged and join the conversation.